Abstract
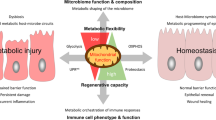
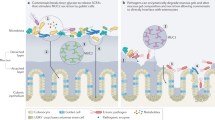
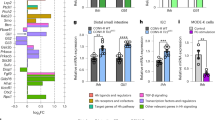
The mammalian intestine houses a complex microbial community, which influences normal epithelial growth and development, and is integral to the repair of damaged intestinal mucosa1–3. Restitution of injured mucosa involves the recruitment of immune cells, epithelial migration and proliferation4,5. Although microenvironmental alterations have been described in wound healing6, a role for extrinsic influences, such as members of the microbiota, has not been reported. Here, we show that a distinct subpopulation of the normal mucosal-associated gut microbiota expands and preferentially colonizes sites of damaged murine mucosa in response to local environmental cues. Our results demonstrate that formyl peptide receptor 1 (FPR1) and neutrophilic NADPH oxidase (NOX2) are required for the rapid depletion of microenvironmental oxygen and compensatory responses, resulting in a dramatic enrichment of an anaerobic bacterial consortium. Furthermore, the dominant member of this wound-mucosa-associated microbiota, Akkermansia muciniphila (an anaerobic, mucinophilic gut symbiont7,8), stimulated proliferation and migration of enterocytes adjacent to the colonic wounds in a process involving FPR1 and intestinal epithelial-cell-specific NOX1-dependent redox signalling. These findings thus demonstrate how wound microenvironments induce the rapid emergence of ‘probiont’ species that contribute to enhanced repair of mucosal wounds. Such microorganisms could be exploited as potential therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA 102, 99–104 (2005).
Lotz, M. M. et al. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am. J. Pathol. 150, 747–760 (1997).
Miyoshi, H., Ajima, R., Luo, C. T., Yamaguchi, T. P. & Stappenbeck, T. S. Wnt5a potentiates TGFβ signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 (2012).
Colgan, S. P., Curtis, V. F. & Campbell, E. L. The inflammatory tissue microenvironment in IBD. Inflamm. Bowel Dis. 19, 2238–2244 (2013).
Derrien, M., Vaughan, E. E., Plugge, C. M. & de Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476 (2004).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Alam, A. et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 7, 645–655 (2014).
Leoni, G. et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123, 443–454 (2013).
Seno, H. et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl Acad. Sci. USA 106, 256–261 (2009).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
Campbell, E. L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014).
Wentworth, C. C., Alam, A., Jones, R. M., Nusrat, A. & Neish, A. S. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J. Biol. Chem. 286, 38448–38455 (2011).
Swanson, P. A. II et al. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc. Natl Acad. Sci. USA 108, 8803–8808 (2011).
Jones, R. M. et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 32, 3017–3028 (2013).
Babbin, B. A. et al. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 179, 8112–8121 (2007).
Reunanen, J. et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of epithelial cell layer. Appl. Environ. Microbiol. 81, 3655–3662 (2015).
Derrien, M. et al. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front. Microbiol. 2, 166 (2011).
Hamady, M., Walker, J. J., Harris, J. K., Gold, N. J. & Knight, R. Error-correcting barcoded primers for pyrosequencing khundreds of samples in multiplex. Nature Methods 5, 235–237 (2008).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of abacteria and archaea. ISME J. 6, 610–618 (2012).
Caporaso, J. G. et al. PyNAST: a flexible tool sfor aligning sequences to a template alignment. Bioinformatics 26, 266–267 (2010).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree: kcomputing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26, 1641–1650 (2009).
Lozupone, C. & Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235 (2005).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Vaishnava, S. et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Derrien, M., Collado, M. C., Ben-Amor, K., Salminen, S. & de Vos, W. M. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 74, 1646–1648 (2008).
Pernthaler, J., Glöckner, F.-O., Schönhuber, W. & Amann, R. in Methods in Microbiology: Marine Microbiology Vol. 30 (ed. Paul, J.) 207–210 (Academic Press, 2001); http://go.nature.com/CdPztu
Kundu, K. et al. Hydrocyanines: a class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew. Chem. Int. Ed. Engl. 48, 299–303 (2009).
Roopchand, D. E. et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 64, 2847–2858 (2015).
Acknowledgements
The authors thank F. David, R. Isett, J. Taylor, H. Yi, M. Zwick and C. Kraft for helpful input. This work was supported by grants RO1DK089763 to A.S.N. and A.N., RO1DK055679 to A.N. and RO1AI64462 to A.S.N. A.A. is supported by a Career Development Award from the Crohn's and Colitis Foundation of America.
Author information
Authors and Affiliations
Contributions
A.A., A.N. and A.S.N. conceptualized the study, directed the work and wrote the manuscript. A.A., G.L. A.N. and A.S.N. planned and analysed the experiments. R.M.J., A.A., G.L., M.Q., H.W., C.D. and H.N. performed the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-8 and relative abundance of the bacterial genus as determined by HTS. (PDF 1379 kb)
Rights and permissions
About this article
Cite this article
Alam, A., Leoni, G., Quiros, M. et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol 1, 15021 (2016). https://doi.org/10.1038/nmicrobiol.2015.21
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2015.21
This article is cited by
-
Helicobacter hepaticus augmentation triggers Dopaminergic degeneration and motor disorders in mice with Parkinson’s disease
Molecular Psychiatry (2023)
-
Gastrointestinal redox homeostasis in ageing
Biogerontology (2023)
-
Oral administration of asparagine and 3-indolepropionic acid prolongs survival time of rats with traumatic colon injury
Military Medical Research (2022)
-
RhoB affects colitis through modulating cell signaling and intestinal microbiome
Microbiome (2022)
-
Early life Lactobacillus rhamnosus GG colonisation inhibits intestinal tumour formation
British Journal of Cancer (2022)