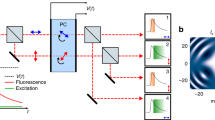
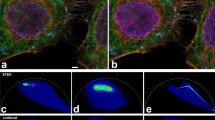
Abstract
We report a substantial signal gain in fluorescence microscopy by ensuring that transient molecular dark states with lifetimes >1 μs, such as the triplet state relax between two molecular absorption events. For GFP and Rhodamine dye Atto532, we observed a 5–25-fold increase in total fluorescence yield before molecular bleaching when strong continuous-wave or high-repetition-rate pulsed illumination was replaced with pulses featuring temporal pulse separation >1 μs. The signal gain was observed both for one- and two-photon excitation. Obeying dark or triplet state relaxation in the illumination process signifies a major step toward imaging with low photobleaching and strong fluorescence fluxes. Please visit methagora to view and post comments on this article
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tsien, R.Y. Imagining imaging's future. Nat. Rev. Mol. Cell Biol. 4 (Suppl.), SS16–SS21 (2003).
Pawley, J.B. (ed.) Handbook of biological confocal microscopy 3rd edn. (Springer, New York, 2006).
Donnert, G. et al. Macromolecular-scale resolution in biological fluorescence microscopy. Proc. Natl. Acad. Sci. USA 103, 11440–11445 (2006).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Tsien, R.Y., Ernst, L. & Waggoner, A. Fluorophores for confocal microscopy: photophysics and photochemistry. in Handbook of biological confocal microscopy, 3rd edn. (ed., Pawley, J.B.) 338–352 (Springer, New York, 2006).
Webb, W.W., Wells, K.S., Sandison, D.R. & Strickler, J. Criteria for quantitative dynamical confocal fluorescence imaging. in Optical Microscopy for Biology (eds Herman, B. & Jacobson, K.) 73–108 (Wiley, New York, 1990).
Conchello, J.-A. & Lichtman, J.W. Optical sectioning microscopy. Nat. Methods 2, 920–931 (2005).
Hell, S.W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated emission depletion microscopy. Opt. Lett. 19, 780–782 (1994).
Brakenhoff, G.J. et al. Real-time two-photon confocal microscopy using a femtosecond, amplified Ti:sapphire system. J. Microsc. 181, 253–259 (1996).
Beaurepaire, E., Oheim, M. & Mertz, J. Ultra-deep two-photon fluorescence excitation in turbid media. Opt. Commun. 188, 25–29 (2001).
Theer, P., Mazahir, H.T. & Denk, W. Two-photon imaging to a depth of 1000 μm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Masters, B.R. et al. Mitigating thermal mechanical damage potential during two-photon dermal imaging. J. Biomed. Opt. 9, 1265–1270 (2004).
Gustafsson, M.G.L. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 102, 13081–13086 (2005).
Eggeling, C., Widengren, J., Rigler, R. & Seidel, C.A.M. Photostability of fluorescent dyes for single-molecule spectroscopy: mechanisms and experimental methods for estimating photobleaching in aqueous solution. in Applied fluorescence in chemistry, biology and medicine. (eds., Rettig, W., Strehmel, B., Schrader, M. & Seifert, H.) 193–240 (Springer, Berlin, 1999).
Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Sanchez, E.J., Novotny, L., Holtom, G.R. & Xie, X.S. Room-temperature fluorescence imaging and spectroscopy of single molecules by two-photon excitation. J. Phys. Chem. A 101, 7019–7023 (1997).
Eggeling, C., Widengren, J., Rigler, R. & Seidel, C.A.M. Photobleaching of fluorescent dyes under conditions used for single-molecule detection: evidence of two-step photolysis. Anal. Chem. 70, 2651–2659 (1998).
Widengren, J., Mets, Ü. & Rigler, R. Fluorescence correlation spectroscopy of triplet states in solution: A theoretical and experimental study. J. Phys. Chem. 99, 13368–13379 (1995).
Patterson, G.H. & Piston, D.W. Photobleaching in two-photon excitation microscopy. Biophys. J. 78, 2159–2162 (2000).
Eggeling, C., Volkmer, A. & Seidel, C.A.M. Molecular photobleaching kinetics of rhodamine 6G by one- and two-photon induced confocal fluorescence microscopy. ChemPhysChem 6, 791–804 (2005).
Kasha, M. Paths of molecular excitation. Radiat. Res. 2 (Suppl.), 243–275 (1960).
Koester, H.J., Baur, D., Uhl, R. & Hell, S.W. Ca2+ fluorescence imaging with pico- and femtosecond two-photon excitation: signal and photodamage. Biophys. J. 77, 2226–2236 (1999).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Acknowledgements
We thank A. Miyawaki (Laboratory for Cell Function and Dynamics, Riken) for supplying the fluorescent protein Venus. We also thank S. Jakobs, S. Verrier and D. Ouw for preparation of the samples, A. Giske, R. Kellner and K. Willig for help with the experimental setup, and V. Westphal and A. Egner for fruitful discussions. Finally, we thank A. Schönle for support with the IMSPECTOR software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Donnert, G., Eggeling, C. & Hell, S. Major signal increase in fluorescence microscopy through dark-state relaxation. Nat Methods 4, 81–86 (2007). https://doi.org/10.1038/nmeth986
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth986
This article is cited by
-
Near-infrared co-illumination of fluorescent proteins reduces photobleaching and phototoxicity
Nature Biotechnology (2023)
-
Systematic study of peak power scaling for an Yb-doped Kerr-lens mode-locked bulk oscillator
Applied Physics B (2023)
-
Optical gearbox enabled versatile multiscale high-throughput multiphoton functional imaging
Nature Communications (2022)
-
Fast optical recording of neuronal activity by three-dimensional custom-access serial holography
Nature Methods (2022)
-
Real-time 3D single molecule tracking
Nature Communications (2020)