Abstract
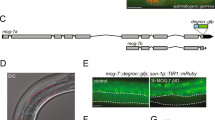
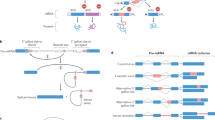
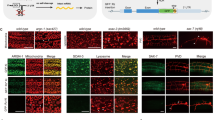
Alternative splicing of pre-mRNAs allows multicellular organisms to create a huge diversity of proteomes from a finite number of genes. But extensive studies in vitro or in cultured cells have not fully explained the regulation mechanisms of tissue-specific or developmentally regulated alternative splicing in living organisms. Here we report a transgenic reporter system that allows visualization of expression profiles of mutually exclusive exons in Caenorhabditis elegans. Reporters for egl-15 exons 5A and 5B showed tissue-specific profiles, and we isolated mutants defective in the tissue specificity. We identified alternative-splicing defective-1 (asd-1), encoding a new RNA-binding protein of the evolutionarily conserved Fox-1 family, as a regulator of the egl-15 reporter. Furthermore, an asd-1; fox-1 double mutant was defective in the expression of endogenous egl-15 (5A) and phenocopied egl-15 (5A) mutant. This transgenic reporter system can be a powerful experimental tool for the comprehensive study of expression profiles and regulation mechanisms of alternative splicing in metazoans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Black, D.L. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell 103, 367–370 (2000).
Maniatis, T. & Tasic, B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature 418, 236–243 (2002).
Stamm, S. et al. Function of alternative splicing. Gene 344, 1–20 (2005).
Eyras, E., Caccamo, M., Curwen, V. & Clamp, M. ESTGenes: alternative splicing from ESTs in Ensembl. Genome Res. 14, 976–987 (2004).
Kampa, D. et al. Novel RNAs identified from an in-depth analysis of the transcriptome of human chromosomes 21 and 22. Genome Res. 14, 331–342 (2004).
Johnson, J.M. et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 302, 2141–2144 (2003).
Pan, Q. et al. Revealing global regulatory features of mammalian alternative splicing using a quantitative microarray platform. Mol. Cell 16, 929–941 (2004).
Sharp, P.A. The discovery of split genes and RNA splicing. Trends Biochem. Sci. 30, 279–281 (2005).
Shin, C. & Manley, J.L. Cell signalling and the control of pre-mRNA splicing. Nat. Rev. Mol. Cell Biol. 5, 727–738 (2004).
Hagiwara, M. Alternative splicing: a new drug target of the post-genome era. Biochim. Biophys. Acta 1754, 324–331 (2005).
Matlin, A.J., Clark, F. & Smith, C.W. Understanding alternative splicing: towards a cellular code. Nat. Rev. Mol. Cell Biol. 6, 386–398 (2005).
Blencowe, B.J. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 25, 106–110 (2000).
Hastings, M.L. & Krainer, A.R. Pre-mRNA splicing in the new millennium. Curr. Opin. Cell Biol. 13, 302–309 (2001).
Xu, X. et al. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell 120, 59–72 (2005).
Lundquist, E.A. et al. The mec-8 gene of C. elegans encodes a protein with two RNA recognition motifs and regulates alternative splicing of unc-52 transcripts. Development 122, 1601–1610 (1996).
Lisbin, M.J., Qiu, J. & White, K. The neuron-specific RNA-binding protein ELAV regulates neuroglian alternative splicing in neurons and binds directly to its pre-mRNA. Genes Dev. 15, 2546–2561 (2001).
Sakharkar, M.K. & Kangueane, P. Genome SEGE: a database for 'intronless' genes in eukaryotic genomes. BMC Bioinformatics 5, 67 (2004).
Nagasaki, H., Arita, M., Nishizawa, T., Suwa, M. & Gotoh, O. Species-specific variation of alternative splicing and transcriptional initiation in six eukaryotes. Gene 364, 53–62 (2005).
Goodman, S.J., Branda, C.S., Robinson, M.K., Burdine, R.D. & Stern, M.J. Alternative splicing affecting a novel domain in the C. elegans EGL-15 FGF receptor confers functional specificity. Development 130, 3757–3766 (2003).
Skipper, M., Milne, C.A. & Hodgkin, J. Genetic and molecular analysis of fox-1, a numerator element involved in Caenorhabditis elegans primary sex determination. Genetics 151, 617–631 (1999).
Gomes, J.E. et al. The maternal gene spn-4 encodes a predicted RRM protein required for mitotic spindle orientation and cell fate patterning in early C. elegans embryos. Development 128, 4301–4314 (2001).
Jin, Y. et al. A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG. EMBO J. 22, 905–912 (2003).
Auweter, S.D. et al. Molecular basis of RNA recognition by the human alternative splicing factor Fox-1. EMBO J. 25, 163–173 (2006).
Lander, E.S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Dell, K.R. & Williams, L.T. A novel form of fibroblast growth factor receptor 2. Alternative splicing of the third immunoglobulin-like domain confers ligand binding specificity. J. Biol. Chem. 267, 21225–21229 (1992).
Baraniak, A.P., Chen, J.R. & Garcia-Blanco, M.A. Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice. Mol. Cell. Biol. 26, 1209–1222 (2006).
Nakahata, S. & Kawamoto, S. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucleic Acids Res. 33, 2078–2089 (2005).
Underwood, J.G., Boutz, P.L., Dougherty, J.D., Stoilov, P. & Black, D.L. Homologues of the Caenorhabditis elegans Fox-1 protein are neuronal splicing regulators in mammals. Mol. Cell. Biol. 25, 10005–10016 (2005).
Mitani, S. Genetic regulation of mec-3 gene expression implicated in the specification of the mechanosensory neuron cell types in Caenorhaabditis elegans. Dev. Growth Differ. 37, 551–557 (1995).
Wicks, S.R., Yeh, R.T., Gish, W.R., Waterston, R.H. & Plasterk, R.H. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28, 160–164 (2001).
Acknowledgements
We thank T. Hirose and T. Fukuhara at Tokyo Medical and Dental University for their discussion. We thank A. Fire at Stanford University School of Medicine, R.Y. Tsien at the University of California, San Diego, H. Nishitoh at TMDU and Caenorhabditis Genetics Center for materials. We thank K. Kawamata, Y. Yamamoto, K. Yamada, G. Ohno and N. Egawa for their technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.K. and M.H.) and Grants-in-aid from National Institute of Biomedical Innovation, Japan (to M.H.).
Author information
Authors and Affiliations
Contributions
H.K. contributed to the overall experiments. T.K. and S.M. contributed to mutant screening and chromosome mapping. H.K. and M.H. organized this work. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Mutation in UGCAUG stretch does not affect E5B-GFP selection in hypodermis. (PDF 87 kb)
Supplementary Fig. 2
Inactivation of E5B-GFP leads to selection of E5A-RFP even though UGCAUG stretch is mutagenized. (PDF 62 kb)
Supplementary Fig. 3
Inactivation of E5B-GFP leads to selection of E5A-RFP in hypodermis. (PDF 83 kb)
Supplementary Fig. 4
Model of regulation of endogenous egl-15 exon 5s in sex myoblasts. (PDF 23 kb)
Supplementary Note 1
Alternative splicing reporter worms with a pair of separate mini-genes. (PDF 94 kb)
Supplementary Note 2
Exon 5B sequence is not required for inclusion or suppresesion of E5B-GFP. (PDF 105 kb)
Rights and permissions
About this article
Cite this article
Kuroyanagi, H., Kobayashi, T., Mitani, S. et al. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nat Methods 3, 909–915 (2006). https://doi.org/10.1038/nmeth944
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth944
This article is cited by
-
Therapeutic manipulation of IKBKAP mis-splicing with a small molecule to cure familial dysautonomia
Nature Communications (2021)
-
Quantitation of the neural silencing activity of anion channelrhodopsins in Caenorhabditis elegans and their applicability for long-term illumination
Scientific Reports (2019)
-
Phosphorylation of the RSRSP stretch is critical for splicing regulation by RNA-Binding Motif Protein 20 (RBM20) through nuclear localization
Scientific Reports (2018)
-
Modulation of aberrant splicing in human RNA diseases by chemical compounds
Human Genetics (2017)
-
Splicing factors control C. elegans behavioural learning in a single neuron by producing DAF-2c receptor
Nature Communications (2016)