Abstract
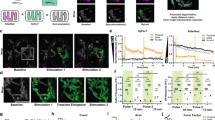
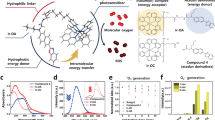
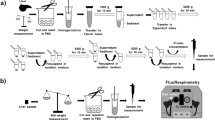
Molecular oxygen is the primary oxidant in biological systems. The ultimate destination of oxygen in vivo is the mitochondria where it is used in oxidative phosphorylation. The ability of this process to produce an amount of high-energy phosphates adequate to sustain life highly depends on the available amount of oxygen. Despite a vast array of techniques to measure oxygen, major (patho)physiological questions remain unanswered because of the unavailability of quantitative techniques to measure mitochondrial oxygen in situ. Here we demonstrate that mitochondrial PO2 can be directly measured in living cells by harnessing the delayed fluorescence of endogenous protoporphyrin IX (PpIX), thereby providing a technique with the potential for a wide variety of applications. We applied this technique to different cell lines (V-79 Chinese hamster lung fibroblasts, HeLa cells and IMR 32-K1 neuroblastoma cells) and present the first direct measurements of the oxygen gradient between the mitochondria and the extracellular volume.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mitchell, P. & Moyle, J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 213, 137–139 (1967).
Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002).
Xi, Q., Cheranov, S.Y. & Jaggar, J.H. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ. Res. 97, 354–362 (2005).
Swartz, H.M. Measuring real levels of oxygen in vivo: opportunities and challenges. Biochem. Soc. Trans. 30, 248–252 (2002).
Vanderkooi, J.M., Erecinska, M. & Silver, I.A. Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am. J. Physiol. 260, C1131–C1150 (1991).
Hogan, M.C. Phosphorescence quenching method for measurement of intracellular PO2 in isolated skeletal muscle fibers. J. Appl. Physiol. 86, 720–724 (1999).
Takahashi, E., Sato, K., Endoh, H., Xu, Z.L. & Doi, K. Direct observation of radial intracellular PO2 gradients in a single cardiomyocyte of the rat. Am. J. Physiol. 275, H225–H233 (1998).
Vanderkooi, J.M., Maniara, G., Green, T.J. & Wilson, D.F. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J. Biol. Chem. 262, 5476–5482 (1987).
Koo, Y.E. et al. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal. Chem. 76, 2498–2505 (2004).
Poulson, R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J. Biol. Chem. 251, 3730–3733 (1976).
Fukuda, H., Casas, A. & Batlle, A. Aminolevulinic acid: from its unique biological function to its star role in photodynamic therapy. Int. J. Biochem. Cell Biol. 37, 272–276 (2005).
Dalton, J., McAuliffe, C.A. & Slater, D.H. Reaction between molecular oxygen and photo-excited protoporphyrin IX. Nature 235, 388 (1972).
Chantrell, S.J., McAuliffe, C.A., Munn, R.W. & Pratt, A.C. Excited states of protoporphyrin IX dimethyl ester: reaction of the triplet with carotenoids. J. C. S. Faraday I 60, 858–865 (1977).
Calvo, M.R. et al. Photophysics of protoporphyrin ions in vacuo: triplet-state lifetimes and quantum yields. J. Chem. Phys. 120, 5067–5072 (2004).
Lo, L.W., Koch, C.J. & Wilson, D.F. Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphine: a phosphor with general application for measuring oxygen concentration in biological systems. Anal. Biochem. 236, 153–160 (1996).
Mik, E.G., Donkersloot, C., Raat, N.J. & Ince, C. Excitation pulse deconvolution in luminescence lifetime analysis for oxygen measurements in vivo. Photochem. Photobiol. 76, 12–21 (2002).
Sudha, B.P., Dixit, N., Moy, V.T. & Vanderkooi, J.M. Reactions of excited-state cytochrome c derivatives. Delayed fluorescence and phosphorescence of zinc, tin, and metal-free cytochrome c at room temperature. Biochemistry 23, 2103–2107 (1984).
Parker, C.A. & Joyce, T.A. Delayed fluorescence and some properties of the chlorophyll triplets. Photochem. Photobiol. 6, 395–406 (1967).
Dunphy, I., Vinogradov, S.A. & Wilson, D.F. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal. Biochem. 310, 191–198 (2002).
Gardner, L.C., Smith, S.J. & Cox, T.M. Biosynthesis of delta-aminolevulinic acid and the regulation of heme formation by immature erythroid cells in man. J. Biol. Chem. 266, 22010–22018 (1991).
Kelty, C.J., Brown, N.J., Reed, M.W. & Ackroyd, R. The use of 5-aminolaevulinic acid as a photosensitiser in photodynamic therapy and photodiagnosis. Photochem. Photobiol. Sci. 1, 158–168 (2002).
Peng, Q. et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 79, 2282–2308 (1997).
Morgan, J. & Oseroff, A.R. Mitochondria-based photodynamic anti-cancer therapy. Adv. Drug Deliv. Rev. 49, 71–86 (2001).
Wilson, B.C., Olivo, M. & Singh, G. Subcellular localization of Photofrin and aminolevulinic acid and photodynamic cross-resistance in vitro in radiation-induced fibrosarcoma cells sensitive or resistant to photofrin-mediated photodynamic therapy. Photochem. Photobiol. 65, 166–176 (1997).
Wu, S.M., Ren, Q.G., Zhou, M.O., Peng, Q. & Chen, J.Y. Protoporphyrin IX production and its photodynamic effects on glioma cells, neuroblastoma cells and normal cerebellar granule cells in vitro with 5-aminolevulinic acid and its hexylester. Cancer Lett. 200, 123–131 (2003).
Gaullier, J.M. et al. Subcellular localization of and photosensitization by protoporphyrin IXhuman keratinocytes and fibroblasts cultivated with 5-aminolevulinic acid. Photochem. Photobiol. 62, 114–122 (1995).
Clark, A., Jr., Clark, P.A., Connett, R.J., Gayeski, T.E. & Honig, C.R. How large is the drop in PO2 between cytosol and mitochondrion? Am. J. Physiol. 252, C583–C587 (1987).
Robiolio, M., Rumsey, W.L. & Wilson, D.F. Oxygen diffusion and mitochondrial respiration in neuroblastoma cells. Am. J. Physiol. 256, C1207–C1213 (1989).
Rick, K. et al. Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood. J. Photochem. Photobiol. B 40, 313–319 (1997).
Van den Boogert, J. et al. 5-Aminolaevulinic acid-induced protoporphyrin IX accumulation in tissues: pharmacokinetics after oral or intravenous administration. J. Photochem. Photobiol. B 44, 29–38 (1998).
Acknowledgements
This work was in part supported by the Technological Collaboration Grant (TSGE 1048) of the Dutch Ministry of Economic Affairs. As part of this collaboration, K. Boller (Department of Laser Physics, University of Twente, Enschede, The Netherlands) kindly provided the pulsed laser system and tunable optical parametric oscillator. A. van Kuilenburg (Laboratory for Genetic Metabolic Diseases, Academic Medical Center, Amsterdam, The Netherlands) kindly provided IMR 32-K1 Neuroblastoma cells. J.S. and J.A.A. were funded in part by a grant from the Dutch Cancer Society (KWF). The authors thank C. van Oven, K. Pos and P. Goedhart for technical assistance.
Author information
Authors and Affiliations
Contributions
E.G.M. conceived and designed the study, performed experiments and wrote the manuscript; J.S. cultured the cells and performed fluorescence microscopy; M.S. contributed to the biological idea; J.F.B. and T.G.L. were involved in the construction of the delayed fluorescence setup; J.A.A. advised on biological experiments; C.I. facilitated the study and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors' institution (The Academic Medical Center in Amsterdam, the Netherlands) has filed a European patent application (number 05076565.0), which covers some of the work described in this article.
Supplementary information
Supplementary Fig. 1
Extended calibration curve in HeLa cells. (PDF 26 kb)
Supplementary Fig. 2
Simultaneous delayed fluorescence and phosphorescence lifetime measurements in a homogenous solution. (PDF 84 kb)
Rights and permissions
About this article
Cite this article
Mik, E., Stap, J., Sinaasappel, M. et al. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods 3, 939–945 (2006). https://doi.org/10.1038/nmeth940
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth940
This article is cited by
-
Relationship between delayed luminescence emission and mitochondrial status in Saccharomyces cerevisiae
Scientific Reports (2022)
-
Understanding delayed fluorescence and triplet decays of Protoporphyrin IX under hypoxic conditions
Photochemical & Photobiological Sciences (2021)
-
Mitochondrial oxygen monitoring with COMET: verification of calibration in man and comparison with vascular occlusion tests in healthy volunteers
Journal of Clinical Monitoring and Computing (2021)
-
Mapping O2 concentration in ex-vivo tissue samples on a fast PLIM macro-imager
Scientific Reports (2020)
-
Synergic pro-apoptotic effects of Ferulic Acid and nanostructured lipid carrier in glioblastoma cells assessed through molecular and Delayed Luminescence studies
Scientific Reports (2020)