Abstract
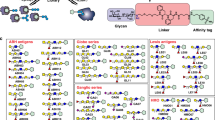
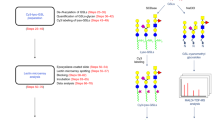
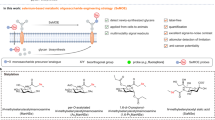
The new field of functional glycomics encompasses information about both glycan structure and recognition by carbohydrate-binding proteins (CBPs) and is now being explored through glycan array technology. Glycan array construction, however, is limited by the complexity of efficiently generating derivatives of free, reducing glycans with primary amines for conjugation. Here we describe a straightforward method to derivatize glycans with 2,6-diaminopyridine (DAP) to generate fluorescently labeled glycans (glycan-DAP conjugates or GDAPs) that contain a primary amine for further conjugation. We converted a wide variety of glycans, including milk sugars, N-glycans, glycosaminoglycans and chitin-derived glycans, to GDAPs, as verified by HPLC and mass spectrometry. We covalently conjugated GDAPs to N-hydroxysuccinimide (NHS)-activated glass slides, maleimide-activated protein, carboxylated microspheres and NHS-biotin to provide quantifiable fluorescent derivatives. All types of conjugated glycans were well-recognized by appropriate CBPs. Thus, GDAP derivatives provide versatile new tools for biologists to quantify and covalently capture minute quantities of glycans for exploring their structures and functions and generating new glycan arrays from naturally occurring glycans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Ratner, D.M. et al. Probing protein-carbohydrate interactions with microarrays of synthetic oligosaccharides. ChemBioChem 5, 379–382 (2004).
Blixt, O. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. USA 101, 17033–17038 (2004).
Adams, E.W. et al. Oligosaccharide and glycoprotein microarrays as tools in HIV glycobiology; glycan-dependent gp120/protein interactions. Chem. Biol. 11, 875–881 (2004).
Feizi, T., Fazio, F., Chai, W. & Wong, C.H. Carbohydrate microarrays—a new set of technologies at the frontiers of glycomics. Curr. Opin. Struct. Biol. 13, 637–645 (2003).
Galanina, O.E., Mecklenburg, M., Nifantiev, N.E., Pazynina, G.V. & Bovin, N.V. GlycoChip: multiarray for the study of carbohydrate-binding proteins. Lab Chip 3, 260–265 (2003).
Ni, J., Singh, S. & Wang, L.X. Synthesis of maleimide-activated carbohydrates as chemoselective tags for site-specific glycosylation of peptides and proteins. Bioconjug. Chem. 14, 232–238 (2003).
Schwarz, M. et al. A new kind of carbohydrate array, its use for profiling antiglycan antibodies, and the discovery of a novel human cellulose-binding antibody. Glycobiology 13, 749–754 (2003).
Nimrichter, L. et al. Intact cell adhesion to glycan microarrays. Glycobiology 14, 197–203 (2004).
Shin, I., Park, S. & Lee, M.R. Carbohydrate microarrays: an advanced technology for functional studies of glycans. Chemistry 11, 2894–2901 (2005).
Wang, D. Carbohydrate microarrays. Proteomics 3, 2167–2175 (2003).
Willats, W.G., Rasmussen, S.E., Kristensen, T., Mikkelsen, J.D. & Knox, J.P. Sugar-coated microarrays: a novel slide surface for the high-throughput analysis of glycans. Proteomics 2, 1666–1671 (2002).
Nakabayashi, S., Warren, C.D. & Jeanloz, R.W. The preparation of a partially protected heptasaccharide-asparagine intermediate for glycopeptide synthesis. Carbohydr. Res. 174, 279–289 (1988).
Likhosherstov, L.M., Novikova, V.A., Dervitskaya, V.A. & Kochetkov, N.K. A new simple synthesis of amino sugar β-D-glycosylamines. Carbohydr. Res. 146, C1–C5 (1986).
Vetter, D. & Gallop, M.A. Strategies for the synthesis and screening of glycoconjugates. 1. A library of glycosylamines. Bioconjug. Chem. 6, 316–318 (1995).
Da Silva, M.L., Tamura, T., McBroom, T. & Rice, K.G. Tyrosine derivatization and preparative purification of the sialyl and asialy-N-linked oligosaccharides from porcine fibrinogen. Arch. Biochem. Biophys. 312, 151–157 (1994).
Likhosherstov, L.M., Novikova, O.S. & Shibaev, V.N. New efficient synthesis of β-glucosylamines of mono- and disaccharides with the use of ammonium carbamate. Dokl. Chem. 383, 89–92 (2002).
Bigge, J.C. et al. Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal. Biochem. 230, 229–238 (1995).
Tomiya, N. et al. Structural analysis of N-linked oligosaccharides by a combination of glycopeptidase, exoglycosidases, and high-performance liquid chromatography. Anal. Biochem. 163, 489–499 (1987).
Locke, D. et al. Neutral, acidic, and basic derivatives of anthranilamide that confer different formal charge to reducing oligosaccharides. Carbohydr. Res. 339, 221–231 (2004).
Watanabe, T., Inoue, N., Kutsukake, T., Matsuki, S. & Takeuchi, M. Labeling conditions using a 2-aminobenzamide reagent for quantitative analysis of sialo-oligosaccharides. Biol. Pharm. Bull. 23, 269–273 (2000).
Gray, G.R. The direct coupling of oligosaccharides to proteins and derivatized gels. Arch. Biochem. Biophys. 163, 426–428 (1974).
Rothenberg, B.E., Hayes, B.K., Toomre, D., Manzi, A.E. & Varki, A. Biotinylated diaminopyridine: an approach to tagging oligosaccharides and exploring their biology. Proc. Natl. Acad. Sci. USA 90, 11939–11943 (1993).
Torres, B.V., McCrumb, D.K. & Smith, D.F. Glycolipid-lectin interactions: reactivity of lectins from Helix pomatia, Wisteria floribunda, and Dolichos biflorus with glycolipids containing N-acetylgalactosamine. Arch. Biochem. Biophys. 262, 1–11 (1988).
Nyame, K., Smith, D.F., Damian, R.T. & Cummings, R.D. Complex-type asparagine-linked oligosaccharides in glycoproteins synthesized by Schistosoma mansoni adult males contain terminal beta-linked N-acetylgalactosamine. J. Biol. Chem. 264, 3235–3243 (1989).
Yago, T. et al. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J. Cell Biol. 158, 787–799 (2002).
Privat, J.P., Delmotte, F., Mialonier, G., Bouchard, P. & Monsigny, M. Fluorescence studies of saccharide binding to wheat-germ agglutinin (lectin). Eur. J. Biochem. 47, 5–14 (1974).
Lee, C.J., Lee, L.H., Lu, C.S. & Wu, A. Bacterial polysaccharides as vaccines–immunity and chemical characterization. Adv. Exp. Med. Biol. 491, 453–471 (2001).
Nyame, A.K., Kawar, Z.S. & Cummings, R.D. Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch. Biochem. Biophys. 426, 182–200 (2004).
Keding, S.J. & Danishefsky, S.J. Prospects for total synthesis: a vision for a totally synthetic vaccine targeting epithelial tumors. Proc. Natl. Acad. Sci. USA 101, 11937–11942 (2004).
Pozsgay, V. Oligosaccharide-protein conjugates as vaccine candidates against bacteria. Adv. Carbohydr. Chem. Biochem. 56, 153–199 (2000).
Nyame, A.K., Leppanen, A.M., DeBose-Boyd, R. & Cummings, R.D. Mice infected with Schistosoma mansoni generate antibodies to LacdiNAc (GalNAc β 1 → 4GlcNAc) determinants. Glycobiology 9, 1029–1035 (1999).
Merkle, R.K. & Cummings, R.D. Lectin affinity chromatography of glycopeptides. Methods Enzymol. 138, 232–259 (1987).
Hase, S. Analysis of sugar chains by pyridylamination. Methods Mol. Biol. 14, 69–80 (1993).
Toomre, D.K. & Varki, A. Advances in the use of biotinylated diaminopyridine (BAP) as a versatile fluorescent tag for oligosaccharides. Glycobiology 4, 653–663 (1994).
Merry, A.H. et al. Recovery of intact 2-aminobenzamide-labeled O-glycans released from glycoproteins by hydrazinolysis. Anal. Biochem. 304, 91–99 (2002).
Acknowledgements
This work was supported by US National Institutes of Health Grant HL065509 to G.P.S. and also supported in part by the Consortium for Functional Glycomics under National Institute of General Medical Sciences, US National Institutes of Health Grant GM62116. We thank A. Lee for help with glycan array analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Examples of glycans used in the study. (PDF 331 kb)
Supplementary Fig. 2
ESI-MS/MS spectra for GlcNAc2-DAP. (PDF 397 kb)
Supplementary Fig. 3
Identification and quantification of DAP on NHS-activated glass slides. (PDF 420 kb)
Supplementary Fig. 4
Free N-glycans analyzed by MALDI-TOF-MS before and after conjugation with DAP. (PDF 319 kb)
Supplementary Fig. 5
A mixture of free high mannose-type N-glycans Man5GlcNAc2 through Man9GlcNAc2. (PDF 329 kb)
Supplementary Fig. 6
Free hyaluronic acid-derived oligosaccharides analyzed by MALDI-TOF-MS before and after conjugation with DAP. (PDF 343 kb)
Supplementary Fig. 7
Free hyaluronic acid-derived oligosaccharides labeled with DAP or 2-AB and analyzed by HPLC. (PDF 270 kb)
Supplementary Fig. 8
Separation of mucin-derived O-glycan GDAPs by HPLC. (PDF 313 kb)
Rights and permissions
About this article
Cite this article
Xia, B., Kawar, Z., Ju, T. et al. Versatile fluorescent derivatization of glycans for glycomic analysis. Nat Methods 2, 845–850 (2005). https://doi.org/10.1038/nmeth808
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth808
This article is cited by
-
“Stuck on sugars – how carbohydrates regulate cell adhesion, recognition, and signaling”
Glycoconjugate Journal (2019)
-
Preparation of glycoconjugates from unprotected carbohydrates for protein-binding studies
Nature Protocols (2017)
-
Rapid and sensitive MALDI MS analysis of oligosaccharides by using 2-hydrazinopyrimidine as a derivative reagent and co-matrix
Analytical and Bioanalytical Chemistry (2017)
-
Oxidative release of natural glycans for functional glycomics
Nature Methods (2016)
-
Glycan microarrays of fluorescently-tagged natural glycans
Glycoconjugate Journal (2015)