Abstract
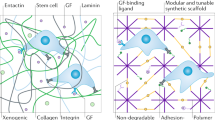
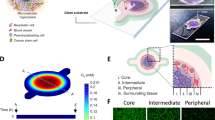
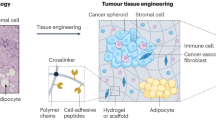
Microenvironmental conditions control tumorigenesis and biomimetic culture systems that allow for in vitro and in vivo tumor modeling may greatly aid studies of cancer cells' dependency on these conditions. We engineered three-dimensional (3D) human tumor models using carcinoma cells in polymeric scaffolds that recreated microenvironmental characteristics representative of tumors in vivo. Strikingly, the angiogenic characteristics of tumor cells were dramatically altered upon 3D culture within this system, and corresponded much more closely to tumors formed in vivo. Cells in this model were also less sensitive to chemotherapy and yielded tumors with enhanced malignant potential. We assessed the broad relevance of these findings with 3D culture of other tumor cell lines in this same model, comparison with standard 3D Matrigel culture and in vivo experiments. This new biomimetic model may provide a broadly applicable 3D culture system to study the effect of microenvironmental conditions on tumor malignancy in vitro and in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bissell, M.J. & Radisky, D. Putting tumours in context. Nat. Rev. Cancer 1, 46–54 (2001).
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Harris, A.L. Hypoxia–a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 (2002).
Debnath, J. & Brugge, J.S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer 5, 675–688 (2005).
Sutherland, R.M. et al. Oxygenation and differentiation in multicellular spheroids of human colon carcinoma. Cancer Res. 46, 5320–5329 (1986).
Debnath, J., Muthuswamy, S.K. & Brugge, J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003).
Lee, G.Y., Kenny, P.A., Lee, E.H. & Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4, 359–365 (2007).
Adam, M.F., Dorie, M.J. & Brown, J.M. Oxygen tension measurements of tumors growing in mice. Int. J. Radiat. Oncol. Biol. Phys. 45, 171–180 (1999).
Zahir, N. et al. Autocrine laminin-5 ligates {alpha}6{beta}4 integrin and activates RAC and NF{kappa}B to mediate anchorage-independent survival of mammary tumors. J. Cell Biol. 163, 1397–1407 (2003).
Yuen, H.W. et al. Suppression of laminin-5 expression leads to increased motility, tumorigenicity, and invasion. Exp. Cell Res. 309, 198–210 (2005).
Sethi, T. et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 5, 662–668 (1999).
Zhang, Y., Lu, H., Dazin, P. & Kapila, Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin {alpha}v mediate survival signals through focal adhesion kinase. J. Biol. Chem. 279, 48342–48349 (2004).
Kerbel, R. & Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2, 727–739 (2002).
Le, Y.J. & Corry, P.M. Hypoxia-induced bFGF gene expression is mediated through the JNK signal transduction pathway. Mol. Cell. Biochem. 202, 1–8 (1999).
Xie, K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 12, 375–391 (2001).
Mizukami, Y. et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat. Med. 11, 992–997 (2005).
Li, A. et al. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis 8, 63–71 (2005).
Christofori, G. New signals from the invasive front. Nature 441, 444–450 (2006).
Mueller, M.M. & Fusenig, N.E. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4, 839–849 (2004).
Janes, S.M. & Watt, F.M. New roles for integrins in squamous-cell carcinoma. Nat. Rev. Cancer 6, 175–183 (2006).
Maschler, S. et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 24, 2032–2041 (2005).
Qian, F., Zhang, Z.C., Wu, X.F., Li, Y.P. & Xu, Q. Interaction between integrin [alpha]5 and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 333, 1269–1275 (2005).
Islam, S., Carey, T.E., Wolf, G.T., Wheelock, M.J. & Johnson, K.R. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J. Cell Biol. 135, 1643–1654 (1996).
Desmouliere, A., Guyot, C. & Gabbiani, G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int. J. Dev. Biol. 48, 509–517 (2004).
dit Faute, M.A. et al. Distinctive alterations of invasiveness, drug esistance and cell-cell organization in 3D-cultures of MCF-7, a human breast cancer cell line, and its multidrug resistant variant. Clin. Exp. Metastasis 19, 161–168 (2002).
Kumar, P. et al. Combination treatment significantly enhances the efficacy of antitumor therapy by preferentially targeting angiogenesis. Lab. Invest. 85, 756–767 (2005).
Ferrara, N., Hillan, K.J. & Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 333, 328–335 (2005).
Alsberg, E., Anderson, K.W., Albeiruti, A., Rowley, J.A. & Mooney, D.J. Engineering growing tissues. Proc. Natl. Acad. Sci. USA 99, 12025–12030 (2002).
Richardson, T.P., Peters, M.C., Ennett, A.B. & Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 (2001).
Harris, L.D., Kim, B.S. & Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 42, 396–402 (1998).
Acknowledgements
We thank K. Polyak (Dana Farber Cancer Institute) for her insightful review of this article, P. Kumar (University of Michigan) for helpful discussions, B. Tilton (Harvard University) for assistance with flow cytometry and Oxford Optronics for support with Oxylite measurements. Financial support was provided by the US National Institutes of Health (RO1 HL069957) and the Deutsche Forschungsgemeinschaft (post-doctoral fellowship to C.F.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Methods (PDF 1273 kb)
Rights and permissions
About this article
Cite this article
Fischbach, C., Chen, R., Matsumoto, T. et al. Engineering tumors with 3D scaffolds. Nat Methods 4, 855–860 (2007). https://doi.org/10.1038/nmeth1085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1085
This article is cited by
-
The progressive trend of modeling and drug screening systems of breast cancer bone metastasis
Journal of Biological Engineering (2024)
-
Breast cancer organoids and their applications for precision cancer immunotherapy
World Journal of Surgical Oncology (2023)
-
Bladder cancer: therapeutic challenges and role of 3D cell culture systems in the screening of novel cancer therapeutics
Cancer Cell International (2023)
-
Breast cancer brain metastasis: from etiology to state-of-the-art modeling
Journal of Biological Engineering (2023)
-
Spheroids, organoids and kidneys-on-chips: how complex human cellular models have assisted in the study of kidney disease and renal ciliopathies
Microfluidics and Nanofluidics (2023)