Abstract
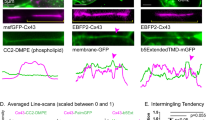
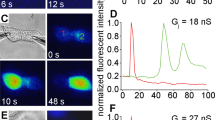
Gap junction channels assembled from connexin protein subunits mediate intercellular transfer of ions and metabolites. Impaired channel function is implicated in several hereditary human diseases. In particular, defective permeation of cAMP or inositol-1,4,5-trisphosphate (InsP3) through connexin channels is associated with peripheral neuropathies and deafness, respectively. Here we present a method to estimate the permeability of single gap junction channels to second messengers. Using HeLa cells that overexpressed wild-type human connexin 26 (HCx26wt) as a model system, we combined measurements of junctional conductance and fluorescence resonance energy transfer (FRET) emission ratio of biosensors selective for cAMP and InsP3. The unitary permeabilities to cAMP (47 × 10−3 ± 15 × 10−3 μm3/s) and InsP3 (60 × 10−3 ± 12 × 10−3 μm3/s) were similar, but substantially larger than the unitary permeability to lucifer yellow (LY; 7 ± 3 × 10−3 μm3/s), an exogenous tracer. This method permits quantification of defects of metabolic coupling and can be used to investigate interdependence of intercellular diffusion and cross-talk between diverse signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sosinsky, G.E. & Nicholson, B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta 1711, 99–125 (2005).
Gerido, D.A. & White, T.W. Connexin disorders of the ear, skin, and lens. Biochim. Biophys. Acta 1662, 159–170 (2004).
Goldberg, G.S., Valiunas, V. & Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta 1662, 96–101 (2004).
Irvine, R.F. 20 years of Ins(1,4,5)P3, and 40 years before. Nat. Rev. Mol. Cell Biol. 4, 586–590 (2003).
Allbritton, N.L., Meyer, T. & Stryer, L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258, 1812–1815 (1992).
Zaccolo, M., Filippin, L., Magalhaes, P. & Pozzan, T. Heterogeneity of second messenger levels in living cells. Novartis Found. Symp. 239, 85–95, 150–159 (2001).
Bos, J.L. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 4, 733–738 (2003).
Bacskai, B.J. et al. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science 260, 222–226 (1993).
Dakin, K., Zhao, Y. & Li, W.H. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat. Methods 2, 55–62 (2005).
Harris, A.L. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 34, 325–472 (2001).
Oh, S. et al. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron 19, 927–938 (1997).
Qu, Y. & Dahl, G. Function of the voltage gate of gap junction channels: selective exclusion of molecules. Proc. Natl. Acad. Sci. USA 99, 697–702 (2002).
Bedner, P. et al. Selective permeability of different connexin channels to the second messenger cyclic AMP. J. Biol. Chem. 281, 6673–6681 (2006).
Saez, J.C., Connor, J.A., Spray, D.C. & Bennett, M.V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc. Natl. Acad. Sci. USA 86, 2708–2712 (1989).
Beltramello, M., Piazza, V., Bukauskas, F.F., Pozzan, T. & Mammano, F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell Biol. 7, 63–69 (2005).
Ponsioen, B. et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 5, 1176–1180 (2004).
Tanimura, A., Nezu, A., Morita, T., Turner, R.J. & Tojyo, Y. Fluorescent biosensor for quantitative real-time measurements of inositol 1,4,5-trisphosphate in single living cells. J. Biol. Chem. 279, 38095–38098 (2004).
Bastianello, S., Ciubotaru, C.D., Beltramello, M. & Mammano, F. in Three-Dimensional and Multidimensional Microscopy: Image Acquisition and Processing XI (ed. Conchello, J.-A.) Vol. 5324, 265–274 (San Jose, California, USA, 2004).
Eckert, R. Gap-junctional single-channel permeability for fluorescent tracers in mammalian cell cultures. Biophys. J. 91, 565–579 (2006).
Valiunas, V., Beyer, E.C. & Brink, P.R. Cardiac gap junction channels show quantitative differences in selectivity. Circ. Res. 91, 104–111 (2002).
Brink, P.R. & Ramanan, S.V. A model for the diffusion of fluorescent probes in the septate giant axon of earthworm: axoplasmic diffusion and junctional membrane permeability. Biophys. J. 48, 299–309 (1985).
Patel, S., Joseph, S.K. & Thomas, A.P. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium 25, 247–264 (1999).
Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 1662, 61–80 (2004).
Paemeleire, K. et al. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol. Biol. Cell 11, 1815–1827 (2000).
Bicego, M. et al. Pathogenetic role of the deafness-related M34T mutation of Cx26. Hum. Mol. Genet. 15, 2569–2587 (2006).
Matsu-ura, T. et al. Cytosolic inositol 1,4,5-trisphosphate dynamics during intracellular calcium oscillations in living cells. J. Cell Biol. 173, 755–765 (2006).
Nikolaev, V.O., Gambaryan, S. & Lohse, M.J. Fluorescent sensors for rapid monitoring of intracellular cGMP. Nat. Methods 3, 23–25 (2006).
Beltramello, M. et al. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem. Biophys. Res. Commun. 305, 1024–1033 (2003).
Downes, C.P., Mussat, M.C. & Michell, R.H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem. J. 203, 169–177 (1982).
Mammano, F. et al. An optical recording system based on a fast CCD sensor for biological imaging. Cell Calcium 25, 115–123 (1999).
Acknowledgements
This work was funded by grants from Telethon Italy (GGP05131) and the European commission FP6 Integrated Project EuroHear (LSHG-CT-20054-512063) under the Sixth Research Frame Program of The European Union (to F.M.) and from Fondazione CARIPARO (to S.P.). M.Z. is supported by Telethon Italy (TCP00089, GGP05113), the Italian Cystic Fibrosis Research Foundation, the Fondazione Compagnia di San Paolo and the HFSPO (RGP1/2005). We thank K. Willecke (University of Bonn), R. Bruzzone (Institute Pasteur), K. Jalink (The Netherlands Cancer Institute) and A. Tanimura (Health Sciences University of Hokkaido) for the gifts of HeLa cells, HCx26wt, H30 and LIBRA, respectively, and T. Pozzan (University of Padova) for helpful discussions and constructive criticism.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Structural models of the HCx26wt connexon and permeant molecules. (PDF 1596 kb)
Rights and permissions
About this article
Cite this article
Hernandez, V., Bortolozzi, M., Pertegato, V. et al. Unitary permeability of gap junction channels to second messengers measured by FRET microscopy. Nat Methods 4, 353–358 (2007). https://doi.org/10.1038/nmeth1031
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1031
This article is cited by
-
Visualization and quantification of dynamic intercellular coupling in human embryonic stem cells using single cell sonoporation
Scientific Reports (2020)
-
The 3.5 ångström X−ray structure of the human connexin26 gap junction channel is unlikely that of a fully open channel
Cell Communication and Signaling (2013)
-
Neurons and β-Cells of the Pancreas Express Connexin36, Forming Gap Junction Channels that Exhibit Strong Cationic Selectivity
The Journal of Membrane Biology (2012)
-
Are GJB2 mutations an aggravating factor in the phenotypic expression of mitochondrial non-syndromic deafness?
Journal of Human Genetics (2010)
-
ATP-mediated cell–cell signaling in the organ of Corti: the role of connexin channels
Purinergic Signalling (2010)