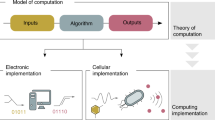
Abstract
Synthetic biologists have advanced the design of trigger-inducible gene switches and their assembly into input-programmable circuits that enable engineered human cells to perform arithmetic calculations reminiscent of electronic circuits. By designing a versatile plug-and-play molecular-computation platform, we have engineered nine different cell populations with genetic programs, each of which encodes a defined computational instruction. When assembled into 3D cultures, these engineered cell consortia execute programmable multicellular full-adder logics in response to three trigger compounds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ma, K.C., Perli, S.D. & Lu, T.K. J. Mol. Biol. 428, 893–915 (2016).
Nissim, L., Perli, S.D., Fridkin, A., Perez-Pinera, P. & Lu, T.K. Mol. Cell 54, 698–710 (2014).
Win, M.N. & Smolke, C.D. Science 322, 456–460 (2008).
Ausländer, S. et al. Nat. Methods 11, 1154–1160 (2014).
Regot, S. et al. Nature 469, 207–211 (2011).
Roquet, N., Soleimany, A.P., Ferris, A.C., Aaronson, S. & Lu, T.K. Science 353, aad8559 (2016).
Tamsir, A., Tabor, J.J. & Voigt, C.A. Nature 469, 212–215 (2011).
Cao, Y. et al. Cell 165, 620–630 (2016).
Schukur, L., Geering, B., Charpin-El Hamri, G. & Fussenegger, M. Sci. Transl. Med. 7, 318ra201 (2015).
Roybal, K.T. et al. Cell 164, 770–779 (2016).
Gitzinger, M., Kemmer, C., El-Baba, M.D., Weber, W. & Fussenegger, M. Proc. Natl. Acad. Sci. USA 106, 10638–10643 (2009).
Gitzinger, M. et al. Nucleic Acids Res. 40, e37 (2012).
Zhou, X., Vink, M., Klaver, B., Berkhout, B. & Das, A.T. Gene Ther. 13, 1382–1390 (2006).
Gossen, M. & Bujard, H. Proc. Natl. Acad. Sci. USA 89, 5547–5551 (1992).
Nissim, L. & Bar-Ziv, R.H. Mol. Syst. Biol. 6, 444 (2010).
Ausländer, D. et al. Mol. Cell 55, 397–408 (2014).
Ausländer, D. et al. Nat. Commun. 5, 4408 (2014).
Rössger, K., Charpin-El Hamri, G. & Fussenegger, M. Proc. Natl. Acad. Sci. USA 110, 18150–18155 (2013).
Hansen, J. et al. Proc. Natl. Acad. Sci. USA 111, 15705–15710 (2014).
Mueller, M. et al. Nat. Chem. Biol. 13, 309–316 (2017).
Schlatter, S. et al. Gene 282, 19–31 (2002).
Acknowledgements
We thank T. Horn, E. Montani and A. Ponti for assistance with microscopy and fluorescence analysis. We are grateful to M. Müller, F. Sedlmayer, D. Fuchs, T. Strittmatter and H. Kim for generous advice; C. Kemmer (Bioversys AG, Basel, Switzerland) for providing plasmids before publication; and D. Fluri (Insphero AG, Zurich, Switzerland) for support with GravityTRAP plates. This work was financially supported in part through a European Research Council (ERC) advanced grant (ProNet, no. 321381) and in part by the National Centre of Competence in Research (NCCR) for Molecular Systems Engineering.
Author information
Authors and Affiliations
Contributions
D.A. and S.A. designed the project. D.A., S.A. and M.F. analyzed the results and wrote the manuscript. D.A., S.A., X.P., L.H. and L.R. performed the experimental work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Electronic full-adder circuit based on NOT-, AND- and OR-logic gates segregated into subprocesses.
a) An electronic full-adder circuit is designed using combinatorial interconnections of NOT-, AND- and OR-logic gates. The circuit integrates the three inputs IA, IB and IC into the two outputs Sum (OS) and Carry (OC). The circuit is segregated into distributed subprocesses illustrated using grey boxes with the corresponding logic description on the left. b) The truth table of the full-adder circuit.
Supplementary Figure 2 Design of a modular logic-gate-based computational platform
a) The AND-logic gate module produces an output only in the presence of both ITF or IR AND ISM or W. The ribozyme-optimized AND-logic gate modules are shown for all three inputs ID, IP and IV and both chemical wires WH and WD. The ANDNOT-logic gate module produces an output only in the presence of ITF ANDNOT ISM. The ribozyme-optimized ANDNOT-logic gate modules responsive to all three inputs ID, IP and IV are shown. b) A dimerization-dependent AND-logic gate module produces an output only in the presence of both IDBD-Coh2 AND IVP16-DocS, resulting in the assembly of a synthetic transcription factor. A DNA-binding domain (DBD) fused to cohesin (Coh2) strongly dimerizes with the dockerin (DocS) fused to a transactivation domain (VP16). The optimized AND-logic gate module using Gal4 as the DBD is shown (purple). Similarly, a dimerization-dependent assembly of a trigger-inducible DBD resulted in an AND ANDNOT-logic gate module that produces an output only in the presence of both IDBD-Coh2 AND IVP16-DocS ANDNOT ISM. The optimized AND ANDNOT-logic gate modules utilizing TetR, VanR and TtgR responsive to all three small-molecule inputs ID, IP and IV are shown. Error bars represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 3 AND-logic gate module optimization using hammerhead ribozymes.
a) Logic description of trigger-inducible AND-logic gate modules responsive to ID, IP and IV with corresponding truth tables. The modules were optimized using relevant inducer combinations as shown using grey boxes (only in the presence of the trigger-inducible transcription factor). b) Performance of three unmodified input-programmable AND-logic gate modules (rtTA/PD, VanR-KRAB/PV-ON, TtgR-KRAB/PP-ON). Two versions of PV-ON-inducible promoters were designed using different constitutive promoters (PSV40 and PhCMV); however, for simplicity, both were named PV-ON. HEK293T cells were engineered with respective AND-logic gate components and induced with corresponding inducer molecules. After 48 h, the activity of the secreted reporter protein SEAP was quantified. OFF-state reporter activity levels are displayed. c) Performance and schematic symbol of ribozyme-optimized AND-logic gate modules, in which hammerhead ribozymes (HHRs) env140 or sTRSV were installed into the 3’-UTR of indicated reporter plasmids. As illustrated, the SEAP levels in the OFF-states are reduced in the presence of HHRs compared to unmodified modules. Partial data are reproduced from Supplementary Fig. 2a. c) Logic description, performance and optimization of the dimerization-dependent AND-logic gate module based on Gal4-Coh2 and VP16-DocS. As illustrated, the introduction of the sTRSV hammerhead ribozyme into the 3’-UTR of the reporter plasmid reduced SEAP levels in the OFF-state. Partial data are reproduced from Supplementary Fig. 2b. Supplementary Table 2 describes the transfection details. Error bars represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 4 ANDNOT-logic gate module optimization using hammerhead ribozymes.
a) Logic description of trigger-inducible ANDNOT-logic gate modules responsive to ID, IP and IV with the corresponding truth table. The modules were optimized using relevant inducer combinations as shown using grey boxes (only in the presence of the trigger-inducible transcription factor). b) Performance of three unmodified input-programmable ANDNOT-logic gate modules (tTA/PD, VanR-VP16/PV-OFF, TtgR-VP16/PP-OFF). HEK293T cells were engineered with respective ANDNOT-logic gate components and induced with corresponding inducer molecules. After 48 h, secreted reporter protein SEAP was quantified. OFF-state activities of the reporter SEAP are displayed. c) Performance and schematic symbol of ribozyme-optimized ANDNOT-logic gate modules in which hammerhead ribozymes (HHRs) env140 or sTRSV were installed into the 3’-UTR of indicated reporter plasmids. As illustrated, the SEAP levels in the OFF-state are reduced in the presence of HHRs compared to unmodified modules. Partial data are reproduced from Supplementary Fig. 2b. d) Performance of minimal promoter-optimized ANDNOT-logic gate modules with replaced minimal promoter (human minimal CMV promoter replaced with a minimal TATA-box) in indicated reporter plasmids. As illustrated, the SEAP levels in the OFF-state are reduced when using a minimal TATA-box compared to unmodified modules. e) Performance and schematic symbol of optimized ANDNOT-logic gate modules with installed HHRs and replaced minimal promoters in indicated reporter plasmids. Supplementary Table S2 describes transfection details. Error bars represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 5 Development and characterization of inter-cellular communication systems based on chemical wires histamine (WH) and dopamine (WD).
a) Logic description, schematic symbol and performance of chemical wire-dependent AND-logic gate modules in HEK293T cells. Cells engineered with the IHRH2 AND WH-logic-gate module function as WH-receiver cells secreting the reporter protein SEAP (OS) in response to WH. Similarly, the IDRD1 AND WD-logic-gate module functions as WD-receiver cells. Partial data are reproduced from Supplementary Fig. 2a. b) Biosynthetic pathway of WH production using histidine decarboxylase (HDC) (left). HEK293T cells were engineered with constitutive expression vectors of a truncated version of histidine decarboxylase (tHDC) or mCherry as control and incubated for 48 h. In a next step, the supernatant was transferred to WH-receiver cells and incubated for another 24 h until SEAP activity was quantified (middle). Different amounts of constitutive tHDC- or mCherry-producing HEK293T cells were cocultured with a fixed WH-receiver cell amount and incubated for 48 h after SEAP activity was quantified in the supernatant of cells (right). c) Biosynthetic pathway of WD production using tyrosine hydroxylase (TH) and aromatic amino acid-decarboxylase (AADC). In addition, GTP cyclohydrolase (GCH1) produces the cofactor tetrahydrobiopterin (BH4) required for TH-dependent catalysis (top). HEK293T cells were engineered with constitutive expression vectors of TH-2A-AADC or mCherry as control and incubated for 48 h. In a next step, the supernatant was transferred to WD-receiver cells and incubated for another 24 h until SEAP activity was quantified (bottom left). Different amounts of constitutive TH-2A-AADC- or mCherry-producing HEK293T cells were cocultured with a fixed WD-receiver cell amount and incubated for 48 h after SEAP was quantified in the supernatant of cells (bottom right). Error bars represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 6 Interoperability and compatibility of logic-gate modules on the inducer molecule and DNA level.
a) Influence of different inducer molecules on input-programmable logic-gate modules. Different AND- and ANDNOT-logic gate modules were transfected into HEK293T cells and exposed to different inducer molecule concentrations (ID, 500 ng/mL doxycycline; IV, 100 μM vanillic acid; IP, 15 μM phloretin; WH, 1 μM histamine; WD, 1 μM dopamine) for 48 h after SEAP activity was quantified in the supernatant of cells. Note the slight reduction of TtgR-VP16-triggered expression in response to IV. b) Crosstalk of different DNA-binding domains on varying DNA operator sequences. Constitutive expression vectors of dimerization-dependent transcription factors (TtgR-Coh2, TetR-Coh2, VanR-Coh2 and Gal4-Coh2) and VP16-DocS were cotransfected with different reporter plasmids encoding inducible promoters with varying DNA operator sequences (PP-OFF, PD, PV-OFF, PG and PCREm). In addition, constitutive expression of HRH2 was used to test the influence of cAMP-activated transcription factors on different inducible promoters when induced with 1 μM of histamine. Error bars represent the normalized mean of n=3 independent experiments performed in triplicate.
Supplementary Figure 7 Implementation of an amplifier in cell I1 improves IV-triggered OS production.
a) A gene circuit design similar to cell I1 is shown, leading to an IV ANDNOT IP ANDNOT ID-dependent production of OS. However, due to the IV-dependent reduction of the ITtgR-Coh2 AND IVP16-DocS ANDNOT IP-logic-gate module (Fig. S6a) for the production of OS, the overall output expression strength (SEAP: 13 U/L) is lower than that of other OS-producing cell types (SEAP: 35-55 U/L; Fig. 2) b) The implementation of an amplifier IGal4-Coh2 AND IVP16-DocS-logic-gate module, implemented downstream of the ITtgR-Coh2 AND IVP16-DocS ANDNOT IP-logic-gate module, enhances OS production (SEAP: 31 U/L) and neutralizes the weakening effects of IV on TtgR-Coh2. Partial data are reproduced from Supplementary Fig. 8b. Error bars represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 8 Nine complex genetic program-engineered human cells perform specific computational subprocesses.
The genetic programs, the truth table and the performance of genetic program-engineered specialized HEK293T cell types and their respective computational subprocesses contributing to the full-adder truth table are shown. a) Two sender cell types, H1 and H2, exclusively produce WH in the presence of ID or IP, respectively, as shown by quantifying the OS of a cocultured WH-responsive sensor cell line. The chemical wire WH acts as activating input for corresponding receiver cells H3 and H4 producing OS only in the presence of WH ANDNOT IV ANDNOT ID (H3) or IP (H4) as shown by quantifying OS production when exposed to respective input combinations. b) The two cell types I1 and I2 are engineered with a three input-programmable genetic program for the production of OS. As shown, cell I1 exclusively produces OS in the presence of IV, while cell I2 requires the presence of all three inputs IDPV to produce OS. c) The two sender cells D1 and D2 are engineered with three input-dependent genetic programs producing WD as output. In contrast, the receiver cell D3 is engineered to exclusively produce OC solely in the presence of WD. Coculturing of cell D3 with either sender cell D1 or D2 in a 3D tissue confirms the logics of the genetic program of D1, which produces WD in the presence of IDP, IDV or IDPV, while D2 secretes WD only in the presence of IPV. Error bars represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 9 Inter-cellular communication devices function more efficiently in a 3D cell culture than in a 2D monolayer setup.
a) HEK293T cells were engineered with genetic programs for cell D1, D2 and D3, and 1600 cells of D1 or 2300 cells of D2 were mixed with 5000 cells of D3 either in a 2D monolayer setup or in a 3D cell culture (untransfected wild-type cells were used to fill the cell mix to 10,000 total cells). Each cell mix was exposed to indicate inducer molecule combinations as shown in the truth table in a 100 μL cell culture volume and cultured for 48 h after secreted luciferase activity was quantified in the supernatant of cells. Partial data are reproduced from Supplementary Fig. 8c. b) HEK293T cells were engineered with genetic programs for cell H1, H2, H3 and H4. The sender cells H1 or H2 were titrated to respective receiver cells H3 and H4 (fixed amount of 6000 cells) and filled to a total of 10,000 cells using untransfected wild-type cells. Each cell mix was exposed to indicated inducer molecules in a 100 μL cell culture volume and cultured for 48 h in either a 2D monolayer setup or in a 3D cell culture after SEAP activity was quantified in the supernatant of cells. The data represent the means ± s.d. of n=3 independent experiments performed in triplicate.
Supplementary Figure 10 3D cell culture formation, z-stack imaging settings and micrograph preparation.
a) Schematic illustration of one well of a 96-well GravityTRAP plate comprising 3D cell culture. The diameter of the bottom of each well is 1 mm. b) Representative brightfield micrographs illustrating the tissue formation process over time. HEK293T cells were seeded onto GravityTRAP plates at time point t=0 and monitored every 6 h until t=48 h. c) Schematic illustration showing the microscopy settings used to image 3D 3D cell culture using a z-stack. The tissue was resolved using 22 slices in z with a distance of 15 μm. d) Representative fluorescent micrographs illustrating 22 slices of an exemplary 3D cell culture and its corresponding maximum intensity projection in z. e) Example of image processing for all fluorescent micrographs shown in the present study using three conditions of the I1-I2 multicellular consortium. Brightfield images are shown, illustrating the 1 mm diameter of the GravityTRAP plate in which the 3D cell culture was cultured and monitored. A square region around the cell culture was cropped, and the cell culture is indicated with a white circle. The original fluorescent micrograph contains a lookup table (LUT) from 1-65,000. The linear LUT was set to 3000-20,000 for all images and fluorescent channels. Scale bars: 100 μm.
Supplementary Figure 11 Three-dimensional multicellular consortia execute higher-order computational logics.
a) The coculture of I1 and I2 cell types forms a 3D multicellular consortium, which performs subprocesses to produce OS exclusively in the presence of IV or IDPV. Each multicellular consortium comprises a total of 10,000 cells, and fluorescence of 3D cell cultures was quantified after 42 h (Error bars represent the means ± s.d. of n=9 3D cell cultures from n=3 independent experiments); Live-cell microscopy experiments quantifying the fluorescence of 3D cell culture every 6 h are illustrated (Error bars represent the means ± s.d. of n=3 3D cell cultures of a representative experiment). Representative fluorescent micrographs illustrating the maximum intensity projections from z-stacks are shown (scale bar, 100 μm) for the coculture of I1 and I2 cell types forms a 3D multicellular consortium, which performs subprocesses to produce OS exclusively in the presence of IV or IDPV. b) The coculture of D1-D3 cell types forms the 3D multicellular consortium, which performs subprocesses to produce OC in the presence of IDP, IDV, IPV or IDPV in a WD-dependent inter-cellular communication process. Each multicellular consortium comprises a total of 10,000 cells, and fluorescence of 3D cell culture was quantified after 42 h (Error bars represent the means ± s.d. of n=9 3D cell cultures from n=3 independent experiments); Live-cell microscopy experiments quantifying the fluorescence of 3D cell culture every 6 h are illustrated (Error bars represent the means ± s.d. of n=3 3D cell cultures of a representative experiment). Representative fluorescent micrographs illustrating the maximum intensity projections from z-stacks are shown (scale bar, 100 μm) for the coculture of D1-D3 cell types forms the 3D multicellular consortium, which performs subprocesses to produce OC in the presence of IDP, IDV, IPV or IDPV in a WD-dependent inter-cellular communication process. c) Representative fluorescent micrographs illustrating the maximum intensity projections from z-stacks are shown (scale bar, 100 μm) for the coculture of H1-H4 cell types form a 3D multicellular consortium, which performs subprocesses to produce OS exclusively in the presence of ID or IP in a WH-dependent inter-cellular communication process.
Supplementary Figure 12 Multicellular consortia H1-H4, I1-I2 and D1-D3 and full-adder 3D cell culture produce secreted reporter genes according to the corresponding truth table of higher-order logic circuits.
a) 3D cell cultures containing cell types H1-H4 secrete the reporter protein SEAP representing OS only in the presence of ID ANDNOT (IP OR IV) OR IP ANDNOT (ID OR IV). The supernatants of the same tissues as shown in Fig. 3a were used to quantify SEAP expression (n=9 tissues examined in three independent experiments). b) 3D cell cultures containing cell types I1-I2 secrete the reporter protein SEAP representing OS only in the presence of IV ANDNOT (ID OR IP) OR ID AND IP AND IV. The supernatants of the same tissues as shown in Fig. 3b were used to quantify SEAP expression (n=9 tissues performed in three independent experiments). c) 3D cell culture containing cells D1-D3 secrete the reporter protein GLuc representing OC in the presence of ID AND (IP OR IV) OR IP AND IV. The supernatants of the same tissues as shown in Fig. 3c were used to quantify GLuc expression (n=9 tissues performed in three independent experiments). d) The full-adder 3D cell culture secretes the reporter proteins SEAP representing OS and GLuc representing OC according to the truth table of the full adder. The supernatants of the same tissues as shown in Fig. 4c were used to quantify SEAP or GLuc expression (n=9 tissues examined in three independent experiments)
Supplementary Figure 13 Representative fluorescent micrographs of full adder 3D cell cultures.
Representative fluorescent micrographs illustrating the maximum intensity projections from z-stacks are shown (scale bar, 100 μm) for the coculture of all nine cell types forms a 3D multicellular consortium, which performs full adder computation to produce OS and OC.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1—13, Supplementary Note 1 and Supplementary Tables 1—4 (PDF 3887 kb)
Life Sciences Reporting Summary
Life Sciences Reporting Summary (PDF 130 kb)
Rights and permissions
About this article
Cite this article
Ausländer, D., Ausländer, S., Pierrat, X. et al. Programmable full-adder computations in communicating three-dimensional cell cultures. Nat Methods 15, 57–60 (2018). https://doi.org/10.1038/nmeth.4505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4505
This article is cited by
-
Engineering intelligent chassis cells via recombinase-based MEMORY circuits
Nature Communications (2024)
-
An electrogenetic interface to program mammalian gene expression by direct current
Nature Metabolism (2023)
-
Engineering a scalable and orthogonal platform for synthetic communication in mammalian cells
Nature Communications (2023)
-
Combinatorial protein dimerization enables precise multi-input synthetic computations
Nature Chemical Biology (2023)
-
Protein-based bandpass filters for controlling cellular signaling with chemical inputs
Nature Chemical Biology (2023)