Abstract
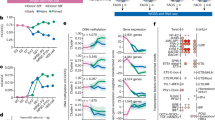
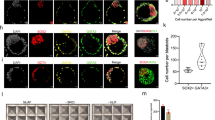
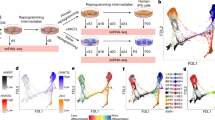
Recent reports on the characteristics of naive human pluripotent stem cells (hPSCs) obtained using independent methods differ. Naive hPSCs have been mainly derived by conversion from primed hPSCs or by direct derivation from human embryos rather than by somatic cell reprogramming. To provide an unbiased molecular and functional reference, we derived genetically matched naive hPSCs by direct reprogramming of fibroblasts and by primed-to-naive conversion using different naive conditions (NHSM, RSeT, 5iLAF and t2iLGöY). Our results show that hPSCs obtained in these different conditions display a spectrum of naive characteristics. Furthermore, our characterization identifies KLF4 as sufficient for conversion of primed hPSCs into naive t2iLGöY hPSCs, underscoring the role that reprogramming factors can play for the derivation of bona fide naive hPSCs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Chan, Y.-S. et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 13, 663–675 (2013).
Gafni, O. et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 (2013).
Takashima, Y. et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269 (2014).
Theunissen, T.W. et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, 471–487 (2014).
Ware, C.B. et al. Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. USA 111, 4484–4489 (2014).
Guo, G. et al. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Reports 6, 437–446 (2016).
Huang, K., Maruyama, T. & Fan, G. The naive state of human pluripotent stem cells: a synthesis of stem cell and preimplantation embryo transcriptome analyses. Cell Stem Cell 15, 410–415 (2014).
Pastor, W.A. et al. Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. Cell Stem Cell 18, 323–329 (2016).
Theunissen, T.W. et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 19, 502–515 (2016).
Sahakyan, A. et al. Human naive pluripotent stem cells model X chromosome dampening and X inactivation. Cell Stem Cell 20, 87–101 (2017).
Vallot, C. et al. XACT noncoding RNA competes with XIST in the control of X chromosome activity during human early development. Cell Stem Cell 20, 102–111 (2017).
Weinberger, L., Ayyash, M., Novershtern, N. & Hanna, J.H. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 17, 155–169 (2016).
Betschinger, J. et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell 153, 335–347 (2013).
Shakiba, N. et al. CD24 tracks divergent pluripotent states in mouse and human cells. Nat. Commun. 6, 7329 (2015).
O'Brien, C.M. et al. New monoclonal antibodies to defined cell surface proteins on human pluripotent stem cells. Stem Cells 35, 626–640 (2017).
Collier, A.J. et al. Comprehensive cell surface protein profiling identifies specific markers of human naive and primed pluripotent states. Cell Stem Cell 20, 874–890.e7 (2017).
Qiu, P. et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat. Biotechnol. 29, 886–891 (2011).
Amir, A.D. et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 31, 545–552 (2013).
Nefzger, C.M. et al. A versatile strategy for isolating a highly enriched population of intestinal stem cells. Stem Cell Reports 6, 321–329 (2016).
Yan, L. et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 20, 1131–1139 (2013).
Okae, H. et al. Genome-wide analysis of DNA methylation dynamics during early human development. PLoS Genet. 10, e1004868 (2014).
von Meyenn, F. et al. Comparative principles of DNA methylation reprogramming during human and mouse in vitro primordial germ cell specification. Dev. Cell 39, 104–115 (2016).
Bock, C. et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Tsankov, A.M. et al. Transcription factor binding dynamics during human ES cell differentiation. Nature 518, 344–349 (2015).
Blakeley, P. et al. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 142, 3151–3165 (2015).
Guo, G. et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 (2009).
Han, D.W. et al. Direct reprogramming of fibroblasts into epiblast stem cells. Nat. Cell Biol. 13, 66–71 (2011).
Wu, J. et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 168, 473–486.e15 (2017).
Yamaguchi, T. et al. Interspecies organogenesis generates autologous functional islets. Nature 542, 191–196 (2017).
Liu, X. et al. Establishing and culturing human naive pluripotent stem cells by primed to naive conversion and reprogramming of fibroblasts. Protocol Exchange http://dx.doi.org/10.1038/protex.2017.099 (2017).
Polanco, J.C. et al. Identification of unsafe human induced pluripotent stem cell lines using a robust surrogate assay for pluripotency. Stem Cells 31, 1498–1510 (2013).
Rooney, D.E. & Czepulkowski, B.H. (Eds.) Human Cytogenetics: A Practical Approach (Oxford University Press, 1992).
Bolger, A.M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G.K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M.D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Law, C.W., Chen, Y., Shi, W. & Smyth, G.K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Li, B. & Dewey, C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Li, B., Ruotti, V., Stewart, R.M., Thomson, J.A. & Dewey, C.N. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 26, 493–500 (2010).
Ritchie, M.E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Tripathi, S. et al. Meta- and orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding. Cell Host Microbe 18, 723–735 (2015).
Polo, J.M. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632 (2012).
Metsalu, T. & Vilo, J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 43, W566–W570 (2015).
Spitzer, M., Wildenhain, J., Rappsilber, J. & Tyers, M. BoxPlotR: a web tool for generation of box plots. Nat. Methods 11, 121–122 (2014).
Enright, A.J. & Ouzounis, C.A. BioLayout—an automatic graph layout algorithm for similarity visualization. Bioinformatics 17, 853–854 (2001).
Guo, W. et al. BS-Seeker2: a versatile aligning pipeline for bisulfite sequencing data. BMC Genomics 14, 774 (2013).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Wells, C.A. et al. Stemformatics: visualisation and sharing of stem cell gene expression. Stem Cell Res. 10, 387–395 (2013).
Acknowledgements
We thank the high-quality cell sorting service and technical input provided by Monash Flowcore Facility and J. Hatwell-Humble for conducting the teratomas experiments. We also thank the Cytogenetics department of Monash Pathology for help with G-banding karyotype analysis. Furthermore, the authors thank the ACRF Centre for Cancer Genomic Medicine at the MHTP Medical Genomics Facility for assistance with next-generation library preparation and Illumina sequencing. This work was supported by National Health and Medical Research Council (NHMRC) project grants APP1104560 to J.M.P. and A.L.L., APP 1069830 to R.L. and a Monash University strategic grant awarded to C.M.N. X.L. was supported by a Monash International Postgraduate Research Scholarship and a Monash Graduate Scholarship. A.S.K. was supported by an NHMRC Early Career Fellowship APP1092280. J.M.P. and R.L. were supported by Silvia and Charles Viertel Senior Medical Research Fellowships.
Author information
Authors and Affiliations
Contributions
X.L., C.M.N. and J.M.P. conceived the study and designed experiments. X.L. performed somatic cell reprogramming in different culture conditions and tissue culture experiments with support from C.M.N., J.C., J.F., K.M., J.M.P. and M.H.-G. S.K.N. supported the teratomas experiments. X.L. and C.M.N. performed FACS experiments, SPADE/viSNE and the molecular experiments of the cells with support from A.S.K. and J.C.; A.S.K., C.H. and R.B.S. performed cell preparation for mass spectrometry experiments and analysis. F.J.R., S.M.W. and C.M.N. analyzed Fluidigm and RNA sequencing data under the guidance of J.M.P. J.P., F.J.R., E.F. and R.L. performed targeted methylation experiments and analyzed the results. N.J. and M.E.B. performed molecular characterization. H.S.C. and C.M.O′B. provided reagents and technical assistance. A.L.L. provided reagents and assisted with writing. X.L., C.M.N. and J.M.P. wrote the manuscript. All authors approved of and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.M.P. is a director in Cell Mogrify; however, the work presented in this manuscript is not related to Cell Mogrify.
Integrated supplementary information
Supplementary Figure 1 Additional characterisation of media converted naive hiPSCs.
(a) Experimental outline for the media conversion approach to toggle primed hiPSCs to a naive state (note: naive t2iLGöY culture condition was insufficient for conversion). (b) Resulting colony morphologies (Scale bar, 250μm) and (c) Immunofluorescence labelling for classical pluripotency markers TRA-1-60, SSEA3, NANOG, OCT4 and naive pluripotency associated markers KLF17 and TFE3 (Scale bar, 25μm).
Supplementary Figure 2 Additional characterisation of different naive hiPSCs.
(a) viSNE map (related to Fig. 1d) coloured for expression levels of cell surface markers CD24, SSEA3, SSEA4, F11R, NLGN4X, TRA-1-60. (b) Histogram of flow cytometry data for detection levels of cell surface marker F11R in primed and naive rt2iLGöY hiPSCs (representative histogram derived from n=3 cell donors 32F, 38F and 55F). (c) MDS plot of primed and different naive hiPSCs derived in this study. Two donors (32F and 55F) were used to generate all the hiPSCs except for cNHSM (one donor, 32F). Each dot represents a different cell culture sample per indicated condition. (d) Growth curves of primed and naive r5iLAF and rt2iLGöY iPSCs (n=3 culture replicates from 32F, error bars, s.e.m.). (e) Bright field image (Scale bar, 250μm) and immunofluorescence staining (Scale bar, 25μm) of primed iPSCs obtained by differentiation of naive rt2iLGöY hiPSCs, (32F).
Supplementary Figure 3 Generation of primed and naive rt2iLGöY hiPSCs via an mRNA reprogramming system.
(a) Schematic representation of experimental design for somatic cell reprogramming via mRNA transfections of human fibroblasts into primed and t2iLGöY hiPSCs. (b) Bright field images (Scale bar, 100μm) and immunofluorescence staining (Scale bar, 25μm) of primed and established rt2iLGöY hiPSCs. (c) Multidimensional representation of flow cytometry data for primed and t2iLGöY hiPSCs reprogrammed by mRNA approach by viSNE map (left) and SPADE tree (right). (d) MDS of primed (one donor, 32F) and t2iLGöY (one donor 32F) hiPSCs reprogrammed by mRNA approach as well as primed (two donors, 32F and 55F) and naive rt2iLGöY hiPSCs (two donors, 32F and 55F) reprogrammed by Sendai approach integrated with a previously published human ICM dataset21. Each dot represents a different cell culture sample per indicated condition.
Supplementary Figure 4 Additional characterisation of naive ct2iLGöY hPSCs generated by overexpression of OSKM or KLF4 alone.
(a) Immunofluorescence labelling of established primed hiPSCs, hESCs, naive ct2iLGöY K hiPSCs and ct2iLGöY K hESCs, Scale bar, 25μm. (b) Table with karyotypes of naive ct2iLGöY K hESCs/hiPSCs at indicated passages. (c) Dome-shaped colony counts of primed hiPSCs transduced with OSKM or KLF4, two passages post transduction (n=3 ± s.e.m., **, p=0.0087). (d) FACS analysis of primed hiPSCs transduced with OSKM or KLF4 at p0, p1, p2, percentages of F11R++/SSEA3/EPCAM+ cells of live cells were recorded (n=3 ± s.e.m., *, p=0.0469 **, p=0.0023). (e) Representative FACS blots of gating strategies used for the analysis depicted in panel d.
Supplementary Figure 5 Additional characterisation of naive ct2iLGöY hPSCs generated by overexpression of OSKM or KLF4 alone.
(a) MDS plot of all datasets (samples generated in this study plus published datasets3-5, 21). Two donors (32F and 55F) were used to generate all the iPSCs except for cNHSM (one donor, 32F), for mRNA reprogramming, primed (one donor, 32F) and t2iLGöY (one donor, 32F) and for primed to t2iLGöY conversion (three donors, H9 ESCs, 32F iPSCs and 38F iPSCs for both ct2iLGöY OSKM and ct2iLGöY K conversion). Each dot represents a different cell culture sample per indicated condition. (b) PCA of single cell data for primed hiPSCs (32 cells), naive rt2iLGöY hiPSCs (41 cells), naive ct2iLGöY OSKM hiPSCs (19 cells) and naive ct2iLGöY K hiPSCs (18 cells); 32F. (c) Schematic representation of experimental design for conversion of primed hPSCs to naive ct2iLGöY hPSCs by transfections with KLF4 mRNAs. (d) Bright field images (Scale bar, 100μm) and immunofluorescence labelling (Scale bar, 25μm) of human naive ct2iLGöY hiPSCs/hESCs generated by mRNA transfections of primed hiPSCs/hESCs using KLF4 mRNAs in t2iLGöY condition.
Supplementary Figure 6 KLF4 facilitates the conversion of primed hPSCs to naive t2iLGöY hPSCs.
(a) Representative FACS blots of analysis of primed cells transduced with or without KLF4 (6 days post transduction) in t2iLGöY medium. (b,c) Combined gene ontology analysis for DEGs (FDR<0.05, LogFC>1) between day 2 samples (with/without KLF4) and for DEGs between day 6 samples (with/without KLF4 in t2iLGöY medium). The contribution of the day 2 and day 6 datasets is indicated in red and blue respectively in panel b and gene ontology categories including FDR are indicated in panel c. (two donors, 32F and 38F) were analysed by RNA sequencing per condition).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1, 2, 4–7.
Supplementary Protocol
Establishment and maintenance of human naive pluripotent stem cells by primed to naive conversion and reprogramming of fibroblasts.
Supplementary Table 3
Proteins detected in primed and naive rt2iLGöY iPSCs by mass spectometry.
Rights and permissions
About this article
Cite this article
Liu, X., Nefzger, C., Rossello, F. et al. Comprehensive characterization of distinct states of human naive pluripotency generated by reprogramming. Nat Methods 14, 1055–1062 (2017). https://doi.org/10.1038/nmeth.4436
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4436
This article is cited by
-
Discordance between chromatin accessibility and transcriptional activity during the human primed-to-naïve pluripotency transition process
Cell Regeneration (2023)
-
Dissecting peri-implantation development using cultured human embryos and embryo-like assembloids
Cell Research (2023)
-
Transient naive reprogramming corrects hiPS cells functionally and epigenetically
Nature (2023)
-
Cell fate roadmap of human primed-to-naive transition reveals preimplantation cell lineage signatures
Nature Communications (2022)
-
NANOS3 downregulation in Down syndrome hiPSCs during primordial germ cell-like cell differentiation
Histochemistry and Cell Biology (2022)