Abstract
Understanding circuit computation in the nervous system requires sampling activity over large neural populations and maximizing the number of features that can be extracted. By combining planar arrays of extracellular electrodes with the three-layered cortex of turtles, we show that synaptic signals induced along individual axons as well as action potentials can be easily captured. Two types of information can be extracted from these signals, the neuronal subtype (inhibitory or excitatory)—whose identification is more reliable than with traditional measures such as action potential width—and a (partial) spatial map of functional axonal projections from individual neurons. Because our approach is algorithmic, it can be carried out in parallel on hundreds of simultaneously recorded neurons. Combining our approach with soma triangulation, we reveal an axonal projection bias among a population of pyramidal neurons in turtle cortex and confirm this bias through anatomical reconstructions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewis, C.M., Bosman, C.A. & Fries, P. Recording of brain activity across spatial scales. Curr. Opin. Neurobiol. 32, 68–77 (2015).
Ahrens, M.B. et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485, 471–477 (2012).
Hell, S.W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Gong, Y. et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 350, 1361–1366 (2015).
Hubel, D.H. & Wiesel, T.N. Receptive fields of single neurones in the cat’s striate cortex. J. Physiol. 148, 574–591 (1959).
Fiscella, M. et al. Recording from defined populations of retinal ganglion cells using a high-density CMOS-integrated microelectrode array with real-time switchable electrode selection. J. Neurosci. Methods 211, 103–113 (2012).
Rios, G., Lubenov, E.V., Chi, D., Roukes, M.L. & Siapas, A.G. Nanofabricated neural probes for dense 3-D recordings of brain activity. Nano Lett. 16, 6857–6862 (2016).
Agarwal, G. et al. Spatially distributed local fields in the hippocampus encode rat position. Science 344, 626–630 (2014).
Pillow, J.W. et al. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 454, 995–999 (2008).
Li, P.H. et al. Anatomical identification of extracellularly recorded cells in large-scale multielectrode recordings. J. Neurosci. 35, 4663–4675 (2015).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2014).
Belkin, D.A. Anoxia: tolerance in reptiles. Science 139, 492–493 (1963).
Connors, B.W. & Kriegstein, A.R. Cellular physiology of the turtle visual cortex: distinctive properties of pyramidal and stellate neurons. J. Neurosci. 6, 164–177 (1986).
Kriegstein, A.R. & Connors, B.W. Cellular physiology of the turtle visual cortex: synaptic properties and intrinsic circuitry. J. Neurosci. 6, 178–191 (1986).
Kriegstein, A.R. Synaptic responses of cortical pyramidal neurons to light stimulation in the isolated turtle visual system. J. Neurosci. 7, 2488–2492 (1987).
Hemberger, M., Pammer, L. & Laurent, G. Comparative approaches to cortical microcircuits. Curr. Opin. Neurobiol. 41, 24–30 (2016).
Shein-Idelson, M., Ondracek, J.M., Liaw, H.-P., Reiter, S. & Laurent, G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 352, 590–595 (2016).
Mulligan, K.A. & Ulinski, P.S. Organization of geniculocortical projections in turtles: isoazimuth lamellae in the visual cortex. J. Comp. Neurol. 296, 531–547 (1990).
Gold, C., Henze, D.A. & Koch, C. Using extracellular action potential recordings to constrain compartmental models. J. Comput. Neurosci. 23, 39–58 (2007).
Budd, J. & Kisvárday, Z.F. (Eds.) Wiring Principles of Cerebral Cortex (Frontiers Media SA, 2015).
Lucas, K. The temperature-coefficient of the rate of conduction in nerve. J. Physiol. 37, 112–121 (1908).
Meeks, J.P. & Mennerick, S. Action potential initiation and propagation in CA3 pyramidal axons. J. Neurophysiol. 97, 3460–3472 (2007).
Pettersen, K.H. & Einevoll, G.T. Amplitude variability and extracellular low-pass filtering of neuronal spikes. Biophys. J. 94, 784–802 (2008).
Henze, D.A. et al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 84, 390–400 (2000).
Prechtl, J.C., Cohen, L.B., Pesaran, B., Mitra, P.P. & Kleinfeld, D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc. Natl. Acad. Sci. USA 94, 7621–7626 (1997).
Senseman, D.M. & Robbins, K.A. High-speed VSD imaging of visually evoked cortical waves: decomposition into intra- and intercortical wave motions. J. Neurophysiol. 87, 1499–1514 (2002).
Clawson, W.P., Wright, N.C., Wessel, R. & Shew, W.L. Adaptation towards scale-free dynamics improves cortical stimulus discrimination at the cost of reduced detection. PLoS Comput. Biol. 13, e1005574 (2017).
Berger, H. Über das Elektrenkephalogramm des Menschen. Arch. Psychiatr. Nervenkr. 87, 527–570 (1929).
Buzsáki, G., Anastassiou, C.A. & Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Bédard, C., Rodrigues, S., Roy, N., Contreras, D. & Destexhe, A. Evidence for frequency-dependent extracellular impedance from the transfer function between extracellular and intracellular potentials: intracellular-LFP transfer function. J. Comput. Neurosci. 29, 389–403 (2010).
Lindén, H., Pettersen, K.H. & Einevoll, G.T. Intrinsic dendritic filtering gives low-pass power spectra of local field potentials. J. Comput. Neurosci. 29, 423–444 (2010).
Lindén, H. et al. Modeling the spatial reach of the LFP. Neuron 72, 859–872 (2011).
Glickfeld, L.L., Roberts, J.D., Somogyi, P. & Scanziani, M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat. Neurosci. 12, 21–23 (2009).
Bazelot, M., Dinocourt, C., Cohen, I. & Miles, R. Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J. Physiol. 588, 2077–2090 (2010).
Swadlow, H.A., Gusev, A.G. & Bezdudnaya, T. Activation of a cortical column by a thalamocortical impulse. J. Neurosci. 22, 7766–7773 (2002).
Stoelzel, C.R., Bereshpolova, Y., Gusev, A.G. & Swadlow, H.A. The impact of an LGNd impulse on the awake visual cortex: synaptic dynamics and the sustained/transient distinction. J. Neurosci. 28, 5018–5028 (2008).
Creutzfeldt, O.D., Watanabe, S. & Lux, H.D. Relations between EEG phenomena and potentials of single cortical cells. I. Evoked responses after thalamic and erpicortical stimulation. Electroencephalogr. Clin. Neurophysiol. 20, 1–18 (1966).
Kreiman, G. et al. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron 49, 433–445 (2006).
Engel, A.K., Fries, P. & Singer, W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716 (2001).
Kang, H. & Schuman, E.M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267, 1658–1662 (1995).
Barthó, P. et al. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 92, 600–608 (2004).
Berényi, A. et al. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. 111, 1132–1149 (2014).
Rossant, C. et al. Spike sorting for large, dense electrode arrays. Nat. Neurosci. 19, 634–641 (2016).
Hilgen, G. et al. Unsupervised spike sorting for large-scale, high-density multielectrode arrays. Cell Rep. 18, 2521–2532 (2017).
Marín, G., Mpodozis, J., Sentis, E., Ossandón, T. & Letelier, J.C. Oscillatory bursts in the optic tectum of birds represent re-entrant signals from the nucleus isthmi pars parvocellularis. J. Neurosci. 25, 7081–7089 (2005).
Taxidis, J., Anastassiou, C.A., Diba, K. & Koch, C. Local field potentials encode place cell ensemble activation during hippocampal sharp wave ripples. Neuron 87, 590–604 (2015).
Hagen, E. et al. Focal local field potential signature of the single-axon monosynaptic thalamocortical connection. J. Neurosci. 37, 5123–5143 (2017).
Ermentrout, G.B. & Kleinfeld, D. Traveling electrical waves in cortex: insights from phase dynamics and speculation on a computational role. Neuron 29, 33–44 (2001).
Blanton, M.G., Lo Turco, J.J. & Kriegstein A.R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J. Neurosci. Methods 30, 203–210 (1989).
Stuart, G.J., Dodt, H.U. & Sakmann, B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 423, 511–518 (1993).
Tateno, T., Jimbo, Y. & Robinson, H.P.C. Spatio-temporal cholinergic modulation in cultured networks of rat cortical neurons: spontaneous activity. Neuroscience 134, 425–437 (2005).
Reinhard, K. et al. Step-by-step instructions for retina recordings with perforated multi electrode arrays. PLoS One 9, e106148 (2014).
Konopacki, J., MacIver, M.B., Bland, B.H. & Roth, S.H. Carbachol-induced EEG 'theta' activity in hippocampal brain slices. Brain Res. 405, 196–198 (1987).
Ernst, C. & Christie, B.R. Isolectin-IB 4 as a vascular stain for the study of adult neurogenesis. J. Neurosci. Methods 150, 138–142 (2006).
Dodt, H.-U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007).
Torr, P.H.S. & Zisserman, A. MLESAC: a new robust estimator with application to estimating image geometry. Comput. Vis. Image Underst. 78, 138–156 (2000).
Brainard, D.H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Quiroga, R.Q., Nadasdy, Z. & Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 16, 1661–1687 (2004).
Marre, O. et al. Mapping a complete neural population in the retina. J. Neurosci. 32, 14859–14873 (2012).
Lagarias, J.C., Reeds, J.A., Wright, M.H. & Wright, P.E. Convergence properties of the Nelder–Mead simplex method in low dimensions. SIAM J. Optim. 9, 112–147 (1998).
Potworowski, J., Jakuczun, W., Lski, S. & Wójcik, D. Kernel current source density method. Neural Comput. 24, 541–575 (2012).
Ness, T.V. et al. Modelling and analysis of electrical potentials recorded in microelectrode arrays (MEAs). Neuroinformatics 13, 403–426 (2015).
Acknowledgements
This work was funded by the Max Planck Society (G.L.), the European Research Council under the EU's 7th framework agreement (FP7/2007-2013)/grant no. 322705 (G.L.), the Minerva Foundation (MS-I), and the Boehringer Ingelheim Fonds (M.H.). We thank M. Klinkmann for help with surgery; A. Arends, A. Macias, T. Manthey, and C. Thum for technical assistance; H. Norimoto for assistance with mammalian slices; A. Umminger, F. Baier, T. Maurer, and G. Schmalbach for mechanical design and fabrication; M. Helmstaedter and P. Bastians (MPI Brain Research) for the block-face scanning electron microscope data in Supplementary Figure 5c; R. Naumann (MPI Brain Research) for the NeuN stain in Figure 1a; T.V. Ness for discussions; S. Reiter, J. Letzkus, and H. Ito for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
M.S.-I., M.H., L.P., and G.L. designed and interpreted the experiments; M.S.I., M.H., and L.P. executed the experiments; M.S.I. analyzed data; M.H. and G.L. performed anatomical reconstructions; M.S.-I., M.H., and L.P. prepared figures; G.L. and M.S.I. prepared the manuscript; G.L. managed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
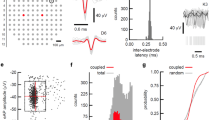
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse.
a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but rates are strongly biased towards low values with an average of 0.04 spikes/s.
b. Average autocorrelation function normalized by the total number of spikes fired by each neuron. Notice low values for all lags indicating rare bursting activity.
c. Average cross-correlation between neurons. For every neuron we calculated the cross-correlation with all simultaneously recorded neurons, normalized to the total number of spikes fired by the neuron. These normalized functions were averaged over the population. Notice low correlation values across all lags.
For all plots, neurons with low SNR (<0.5), average spike amplitude (<10μV) or low number of spikes (<200) were rejected from this analysis.
Supplementary Figure 2 Scalability to large, high-density CMOS electrode arrays.
a. Bright-field image of a cortical slab on a CMOS electrode array. The large size of the active area (2.1mm x 3.85mm), enables recording from very large slabs of intact cortex with high spatial resolution (pitch = 17.5 μm).
b. High-pass filtered spike template (10ms window starting 1.5ms before spike time) over 1024 simultaneously recorded electrodes. Template was averaged over 500 consecutive spikes identified in one channel as negative peaks exceeding 5.5 median absolute deviations.
c. Spike-triggered average of raw voltage traces over the spikes and electrodes in b (200ms window starting 100ms before spike time). The positive amplitude of each trace is color-coded. Note the dominant positive slow SIFs following the spike waveform.
Supplementary Figure 3 Simultaneous patch-MEA recordings.
a. Schematic drawing of recording configuration with combining intracellular whole-cell patch-clamp (black pipette) and extracellular MEA recording (grid). Cortical slabs are typically placed on the MEA ventricular face down.
b. Simultaneous whole-cell patch-clamp (upper black trace) and MEA (lower red trace) recordings of current-induced action potentials in one interneuron. Lower trace: step current injection.
c. Correspondence between average intracellular and extracellular spike waveforms.
Supplementary Figure 4 Robustness of triangulation of soma position and comparison of exponential to power-law decay model.
a. Spatial spread of extracellular spike signal from a sorted unit. Each box represents the mean (red) and individual traces (grey; see look-up-table at right; P = probability of occurrence over 250 consecutive spikes) originating from source location (blue dot at center) in x,y plane. Vertical gray line indicates spike peak time (t=0). Electrode pitch: 40μm. The somatic and axonal signals differ both in shape (bi or tri-phasic for axonal, see top row) and timing (note delays in the axonal negative peak. Note that soma triangulation is not affected by the bias introduced by the axon.
b. Spatial exponential decay (λ = 42μm) of peak spike amplitude (data from neuron in a). Distance measured in x,y,z from position of source, itself computed by triangulation. Red curve fitted to points in black. Data points corresponding to the axonal signal (negative potentials) do not affect the fitting parameters and the resulting exponential curve.
c. Comparison of fitting error between exponential (Exp; "V=" "V" _"0" "e" ^("-" x/λ)) and power law (Pow; "V=" "V" _"0" "x" ^("-" λ)) models for spatial decay of spike amplitude from the soma. The central mark in the box plot indicates the median, and the bottom and top edges of the box indicate the 1st (Q1) and 3rd (Q3) quartiles, respectively (148 neurons; 3 preparations). Whiskers at [Q1-1.5*(Q3-Q1), Q3+1.5*(Q3-Q1)].
Supplementary Figure 5 Propagation of axonal spikes.
a. Left: An example of the spatial spread of the averaged (unfiltered) spike waveform across the different electrodes. Notice the tri-phasic axonal-like signal near top right. A negative field is visible following the spike. Right: timing of spike at different electrodes (red circles) is determined from the negative peak of the highpass (>200Hz) waveform after smoothing with a median filter of 0.35ms. Only significant peaks with peak prominence > 1 (findpeaks, Matlab), width at half prominence > 0.5ms and absolute peak height > 5*std are shown (std is the standard deviation during 1.5-4ms before spike peak). The temporal shift in spike peak is clearly visible.
b. Estimation of axonal propagation velocity. The distance of every electrode from the triangulated soma position is plotted as a function of the corresponding spike-peak delay for all significant peaks. To calculate the velocity, a least square fit is performed and Cook’s distances are calculated. The final speed is calculated by refitting over data points not rejected. Rejected (red circles) are points with a Cook distance larger than median + 0.5*MAD of the Cook distances.
c. Left: scanning electron microscope image from a block scan of turtle cortex. Inset shows an enlargement of area marked by the red square. One of the axons in the image (white arrow) was traced from its cell body by serial block-face scanning, and its diameter calculated as a function of distance from the cell body (right: blue) and converges to a diameter of <500nm. The axon of a different cell is shown in red.
d. Distribution of propagation velocities calculated (as in b) across neurons. To select only neurons for which propagation can be accurately calculated we considered only those with enough (>20) significant peaks at distances >300μm from the triangulated cell body position and with a p-value < 10-4 for the speed term in the linear regression (in total, 821 neurons). Red line marks the average value (153μm/ms).
Supplementary Figure 6 Fine characterization of SIF sub-types.
a. Ordered correlation matrix of SIF waveforms from 1411 separated units (see methods) obtained from seven cortex preparations. The blue rectangle corresponds to excitatory cells; the red, purple and green rectangles correspond to inhibitory cells with SIFs of different amplitudes and delays (as shown in b).
b. Average waveforms of each one of the clusters in a. Initial negativity at t = 0 represents the action potential. Templates with positive SIFs differ in their amplitude as well as kinetics, possibly due, in some cases, to contamination by polysynaptic effects.
c. Correspondence between SIF amplitude, polarity and spike width for different SIF templates. Spike width was measured as the width at half the maximal amplitude on the electrode with the maximal spike amplitude on the array.
d. SIFs yield better type separation than spike width. Red and blue correspond to inhibitory and excitatory neurons classified as in Fig 3. SIF score was determined by first extracting, for each electrode, the maximal correlation value between all templates and all electrodes with distances of up to 290μm from the spike peak electrode. Finally the score was calculated as the sum over the correlation values to inhibitory templates minus the correlation values to excitatory templates.
e. Frequency (probability) distribution of correlation between each spike waveform and I1 template waveform (red in b) after removing cluster I2 (purple in a-c, <18% of all neurons). Two clear modes can be identified, corresponding to the E and I populations. Only a small fraction of the waveforms (1.9%, grey band) show low correlation values that preclude clear classification.
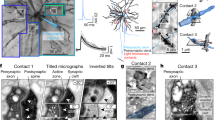
Supplementary Figure 7 Intrinsic electrical properties, morphology and postsynaptic effect identify neuron types.
Patched neurons in turtle cortex could be classified as excitatory or inhibitory using electrophysiological criteria, such as spike width, firing frequency and firing frequency adaptation, as well as morphological parameters such as the presence (in excitatory neurons) or absence (in inhibitory neurons) of dendritic spines. As ground truth we used evoked monosynaptic postsynaptic potentials/currents in paired recordings. The ground truth data suggests a very good match with the morphological feature of spiny vs. aspiny dendrites, which were thus used when no paired recordings were performed.
a. Excitatory neurons. a1: Current-induced spike train in an excitatory neuron. Note the typical adaptation of spike frequency, long action potential half-width and spikelets. a2: Morphology of the neuron in a1, stained with biocytin and viewed from the ventricular side of cortex. Arrows point to segments shown in insets. Insets: zoom-ins on dendritic (top) and axonal (bottom) segments. a3: Images of another stained excitatory cell with clear spiny dendrites (right).
b. Inhibitory neurons. Same as in a but for two cortical interneurons (b2: layer 3; b3: layer 1). Note facilitation of spike frequency and short AP half-width. Dendrites appear aspiny and axonal bouton density is higher than in pyramidal neurons.
c. Spiny pyramidal neurons have excitatory postsynaptic effects. c1: Current induced spiking of a pyramidal neuron elicited excitatory postsynaptic potentials (epsps) in a simultaneously recorded spiny pyramidal neuron. Six trials of the presynaptic Vm were aligned to the peak of the first action potential (vertical broken grey line), overlaid and are shown together with the average Vm (grey). Postsynaptic responses and the average (blue) epsp are shown below. c2: Schematic connectivity (left) and morphologies of the two cells recorded in c1 (tangential view).
d. Scatterplot of the action potential half-width versus adaptation ratio for 282 cortical neurons (grey dots). Red dots and blue triangles show neurons with inhibitory and excitatory postsynaptic effect, respectively. Neurons with excitatory effect had spiny dendrites, while neurons with inhibitory effects had aspiny smooth dendrites.
Supplementary Figure 8 Suppression of all spatial SIF components following synaptic block.
a. A pyramidal neuron was patched and its extracellular action potential and SIF waveforms were recorded on a planar MEA before (gray) and after (red) application of synaptic blockers. A top view of the reconstructed axons is superimposed in magenta. Note that, whereas the SIF is abolished following synaptic block, the spike waveform is unaffected.
b. Same as (a) for a different pyramidal neuron.
Supplementary Figure 9 Correspondence between spatial distribution of SIFs and axonal projections.
Pyramidal neurons were patched and their extracellular action potential and SIF waveforms (black traces, right column) were recorded on a planar MEA (120 electrodes and 100μm pitch in a and c, 59 electrodes and 200μm pitch in b). The neuron was stained by intracellular injection of biocytin, reconstructed, and its morphology aligned to the MEA. Top: perspective from one side to see depth of axonal projections. Bottom: top view of the neuron on the array’s x,y plane. Dendrite shown in grey. Axon collaterals shown in color code, where hue represents distance from electrode array. In all cases, SIF amplitudes are larger where axon collaterals run close to the MEA electrodes.
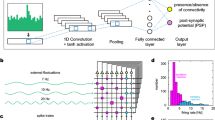
Supplementary Figure 10 SIF dynamics.
Current-source density analysis applied to spike-sorted data in the x-y plane (details in Methods) for 16 neurons (8 interneurons, top; 8 excitatory cells, bottom) recorded simultaneously. Blue shading: negative or inward (“sink”); red shading: positive or outward (“source”). 0ms is time of action potential’s negative peak. Cross-hair on triangulated soma position.
Supplementary Figure 11 Dependence of SIF spatial distribution on network firing rates.
a. Identification of high and low network firing epochs. The high-frequency activity intensity (AI) was extracted and high-activity events were detected (grey, see Methods) and separated from low-activity events (white).
b. Examples of SIFs of two neurons (top and bottom rows) calculated from spikes fired during low (left) and high (right) activity epochs. SIF morphology can be either insensitive (top row) or sensitive (bottom row) to activity levels, as evident by the high (0.99) or low (0.21) spatial correlation value between SIFs, respectively. Intersection of red lines marks the triangulated position of the cell body in x,y plane.
c. The distribution of the relative (absolute value normalized to the maximal amplitude) peak amplitude difference between low and high activity epochs. For most (more than half) neurons, the relative difference is below 20%).
d. The distribution of the spatial correlation between SIFs during high and low activity levels. For most neurons (more than half), the correlation value is above 0.7.
In c and d, only neurons (n=808) for which SIFs could be accurately estimated (fired at least 250 spikes during both high and low activity epochs) were considered.
Supplementary Figure 12 Reliability of orientation-bias detection.
The reliability of SIF orientation-bias detection was tested against potential measurement or analytical artifacts.
In a and b, we rejected all neurons contained in a 300μm-wide inner ring starting at the edge of the MEA, thus precluding artifacts linked to biased sampling of neurons at the edges (N=147 excitatory neurons, 531 interneurons).
In c and d, we rejected all neurons with spike amplitude <10μV, nspikes<200 and SNR<0.5 (see methods) (N=151 excitatory neurons, 646 interneurons).
In e and f, we rejected all neurons with positive, low amplitude SIFs (cluster I2 in Suppl. Fig. 6b, purple) (N=150 excitatory neurons, 651 interneurons from the 1411 neurons analyzed in Suppl Fig 6).
In g and h, we extracted SIFs only from spikes fired during low network activity epochs. Note the remaining bias in excitatory projections, and the reduction of inhibitory projection fields, presumably linked to the absence of compound polysynaptic SIFs. Neurons with low SNR (<0.5), average spike amplitude (<5μV) or low number of spikes (<100) were rejected. (N=108 excitatory neurons, 114 interneurons).
a,c,e,g: Density plot of SIF vector distribution in Cartesian coordinates (as in Fig. 5c) pooled over 7 preparations for inhibitory (left) and excitatory (right) neurons.
b,d,f,h: Average (interpolated) SIFs over 7 preparations for inhibitory (left) and excitatory (right) neurons (as in Fig. 5d).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12. (PDF 2344 kb)
Life Sciences Reporting Summary
Life Sciences Reporting Summary. (PDF 158 kb)
Supplementary Software
SIF analysis. Matlab routine for extracting SIFs from multi electrode extracellular recordings. (ZIP 17343 kb)
Rights and permissions
About this article
Cite this article
Shein-Idelson, M., Pammer, L., Hemberger, M. et al. Large-scale mapping of cortical synaptic projections with extracellular electrode arrays. Nat Methods 14, 882–890 (2017). https://doi.org/10.1038/nmeth.4393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4393
This article is cited by
-
Positive and biphasic extracellular waveforms correspond to return currents and axonal spikes
Communications Biology (2023)
-
Functional neuronal circuitry and oscillatory dynamics in human brain organoids
Nature Communications (2022)
-
High-density electrode recordings reveal strong and specific connections between retinal ganglion cells and midbrain neurons
Nature Communications (2022)
-
A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons
Nature Biomedical Engineering (2019)
-
Multielectrodes join the connectome
Nature Methods (2017)