Abstract
We improve multiphoton structured illumination microscopy using a nonlinear guide star to determine optical aberrations and a deformable mirror to correct them. We demonstrate our method on bead phantoms, cells in collagen gels, nematode larvae and embryos, Drosophila brain, and zebrafish embryos. Peak intensity is increased (up to 40-fold) and resolution recovered (up to 176 ± 10 nm laterally, 729 ± 39 nm axially) at depths ∼250 μm from the coverslip surface.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aviles-Espinosa, R. et al. Biomed. Opt. Express 2, 3135–3149 (2011).
Rueckel, M., Mack-Bucher, J.A. & Denk, W. Proc. Natl. Acad. Sci. USA 103, 17137–17142 (2006).
Tao, X. et al. Opt. Express 20, 15969–15982 (2012).
Ji, N., Sato, T.R. & Betzig, E. Proc. Natl. Acad. Sci. USA 109, 22–27 (2012).
Wang, K. et al. Nat. Commun. 6, 7276 (2015).
Wang, K. et al. Nat. Methods 11, 625–628 (2014).
York, A.G. et al. Nat. Methods 9, 749–754 (2012).
Ingaramo, M. et al. Proc. Natl. Acad. Sci. USA 111, 5254–5259 (2014).
De Luca, G.M. et al. Biomed. Opt. Express 4, 2644–2656 (2013).
Sheppard, C.J.R. Optik 80, 53–54 (1988).
Müller, C.B. & Enderlein, J. Phys. Rev. Lett. 104, 198101 (2010).
Schulz, O. et al. Proc. Natl. Acad. Sci. USA 110, 21000–21005 (2013).
Roth, S., Sheppard, C.J.R., Wicker, K. & Heintzmann, R. Opt. Nanoscopy 2, 5 (2013).
York, A.G. et al. Nat. Methods 10, 1122–1126 (2013).
Winter, P.W. et al. Optica 1, 181–191 (2014).
Winter, P.W. et al. Opt. Express 23, 5327–5334 (2015).
Hofer, H., Artal, P., Singer, B., Aragón, J.L. & Williams, D.R. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 18, 497–506 (2001).
Luo, W., Lieu, Z.Z., Manser, E., Bershadsky, A.D. & Sheetz, M.P. PLoS One 11, e0163915 (2016).
Clokey, G.V. & Jacobson, L.A. Mech. Ageing Dev. 35, 79–94 (1986).
Huang, F. et al. Cell 166, 1028–1040 (2016).
Gould, T.J., Burke, D., Bewersdorf, J. & Booth, M.J. Opt. Express 20, 20998–21009 (2012).
Thomas, B., Wolstenholme, A., Chaudhari, S.N., Kipreos, E.T. & Kner, P. J. Biomed. Opt. 20, 026006 (2015).
Gonsalves, R.A. Opt. Eng. 21, 215829 (1982).
Paxman, R.G., Schulz, T.J. & Fienup, J.R. J. Opt. Soc. Am. A 9, 1072–1085 (1992).
Kner, P. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 30, 1980–1987 (2013).
Débarre, D. et al. Opt. Lett. 34, 2495–2497 (2009).
Wu, Y. et al. Optica 3, 897–910 (2016).
Fischer, R.S., Gardel, M., Ma, X., Adelstein, R.S. & Waterman, C.M. Curr. Biol. 19, 260–265 (2009).
Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B. & Schilling, T.F. Dev. Dyn. 203, 253–310 (1995).
Acknowledgements
We thank P. Clemenceau, G. Clouvel, J. Legrand, A. Jasaitis, and the entire Imagine Optic team for many helpful discussions about adaptive optics and the use of their DM and SHS. We thank J. Tam (NEI) for useful discussions about AO, H. Eden (NIBIB) for helpful comments on the manuscript, and X. Chen (NIDDK) for help in preparing polyacrylamide gel samples. Support for this work was provided by the Intramural Research Programs of the National Institute of Biomedical Imaging and Bioengineering; the National Institute of Heart, Lung, and Blood; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Key Basic Research (973) Program of China grant (2015CB755502); the National Natural Science Foundation of China grant (81471702); the Shenzhen Science and Technology Innovation Committee grant (KQCX20120816155352228); and NIH grant R01MH107238 to D.B.A. The NIH, its officials, and its employees do not recommend or endorse any company, product, or service.
Author information
Authors and Affiliations
Contributions
P.W. and H.S. conceived the project. W.Z., Y.W., P.W., and H.S. designed the optical system. W.Z., Y.W., and P.W. built the optical system and wrote software. W.Z. acquired data. W.Z., Y.W., and H.S. analyzed data. W.Z., P.W., R.F., D.D.N., A.H., C.M., and R.C. prepared samples. R.F., D.D.N., A.H., C.M., R.C., J.Z., A.C., J.S., and C.W. provided advice on biological samples. W.P.D. and D.B.A. contributed reagents and protocols. W.Z. and H.S. wrote the paper with advice from all authors. H.S. supervised research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
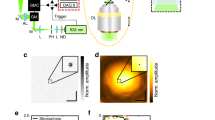
Supplementary Figure 1 Schematic of 2P-ISIM AO optical setup.
Excitation from a femtosecond laser is passed through intensity control and shuttering optics (1/2 λ wave plate, polarizing beam splitting cube, and electro-optic modulator, EOM), spatially filtered (pinhole, 100 μm diameter), beam expanded 5-fold (lens pair L1 and L2, f1 = 40 mm, f2 = 200 mm; and iris) and directed onto a 2D galvanometric mirror (excitation scanner). The point between each galvo surface is made conjugate to a deformable mirror, via lens pair L3 and L4 (f3, f4 = 250 mm). Lens pair L5 and L6 (f5, f6 = 200 mm) images the deformable mirror onto the back focal plane of a 1.2 NA water objective, thereby creating diffraction limited excitation at the sample. Fluorescence is collected along the same path until a dichroic mirror, placed between the excitation scanner and L3, diverts it onto a second 2D galvanometric mirror (emission scanner). Lens pair L4 and L3 images the back focal plane of the objective onto this scanner, which serves to ‘rescan’ fluorescence emission before imaging onto an electron multiplying CCD (EM-CCD) via lens L7 (f7 = 250 mm), enabling super-resolution imaging. Alternatively, for wavefront measurement, the emission scanner is set to descan the fluorescence, and a flip mirror directs this descanned signal onto a Shack Hartmann sensor. Lens pair L8 and L9 (f8 = 250 mm, f9 = 100 mm) serve to image the point between galvo surfaces in the emission scanner onto the Shack Hartman sensor, thus mapping the back focal plane of the objective onto this sensor. Note that lens pairs L1 and L2; L3 and L4; L5 and L6; and L8 and L9 are placed in 4f imaging configurations, thereby ensuring that the back focal plane of the objective, galvanometric scanners, and deformable mirror are conjugate planes. Filters F1 and F2 serve to reject excitation from reaching the EM-CCD and SHS, respectively. See Methods, Supplementary Table 2 for more detail.
Supplementary Figure 2 AO improves performance of 2P-ISIM in aberrated phantom samples.
a) 200 nm yellow green fluorescent beads were placed within the inner surface of a curved coverslip and imaged without b) and with c) AO correction, and d) subsequently deconvolved. Lateral (top) and axial (bottom) views are shown, as are corresponding wavefront maps (root-mean square, RMS, wavefront error 0.222 μm before AO correction (c), 0.030 μm after AO, (d)). e) Lateral (top) and axial (bottom) line profiles as indicated in b-c) indicate a 40-fold increase in peak signal (note that profiles without AO have been magnified 10-fold for clarity). See also Supplementary Fig. 4. For a second test, we embedded 100 nm (small circles) and 1 μm (larger circles) yellow green fluorescent beads within a polyacrylamide gel f). 1 μm beads were used for AO correction due to their strong fluorescence signal, and the nearby 100 nm beads were used for system resolution measurement. Lateral (h) and axial (i) images through representative beads at indicated depth alternately show images without (odd images) and with (even images) AO correction and subsequent deconvolution. Spherical aberration (g) due to the refractive index mismatch increases with depth, and is mitigated using AO at all depths, see also Supplementary Fig. 5. Scale bars: 0.5 μm.
Supplementary Figure 3 Estimating system resolution at coverslip surface in AO-2P ISIM.
a) Exemplary images of 100 nm yellow-green fluorescence beads on coverslip surface, as viewed in lateral (top) and axial (bottom) cuts through the image stacks. From left to right: two-photon wide-field (non-rescan) image, two-photon wide field image after deconvolution, two-photon ISIM image (i.e. after rescanning), two-photon ISIM image after deconvolution. System aberrations were corrected by measuring the wavefront distortion using a Shack-Hartmann sensor, and subsequently compensating for the aberrated wavefront by using a deformable mirror. b) Modulation transfer functions corresponding to a). c) Comparative line-profiles through the center of each lateral (left) and axial (right) bead image further emphasize improvement after deconvolution. (d) Statistical comparison of estimated lateral (left) and axial (right) resolution (FWHM of bead image), derived from > 30 beads. Means and standard deviations are shown. Scale bars: 0.5 μm.
Supplementary Figure 4 Correcting aberrations through a curved glass surface with AO-2P ISIM.
a) Raw (left) and AO-corrected (right) 2P-ISIM images of a 200 nm bead at the curved surface. b) Corresponding images of wavefront measured by Shack-Hartmann sensor. RMS wavefront error is 0.222 μm without AO, and 0.030 μm with AO. c) Decomposition of wavefront into aberration modes (representative Zernike modes shown at right), indicating substantial astigmatism and coma. See also Supplementary Fig. 2. Scale bars: 0.5 μm.
Supplementary Figure 5 Correcting aberrations through a polyacrylamide gel.
a) 2P ISIM fluorescence (pre-AO correction) image measured at depth of 200 μm above the coverslip. The strong fluorescence from 1 μm beads (arrowhead) was used for AO correction and the nearby 100 nm beads (arrows) were used for resolution assessment. b) Decomposition of wavefront into Zernike aberration modes, indicating substantial spherical aberration due to refractive index mismatch. Aberration modes are color-coded according to depth in polyacrylamide gel. c) Representative lateral slices (top) and corresponding wavefront maps (bottom) through 100 nm yellow green beads 50 μm into gel, indicating apparent size of bead without (left) and with (right) correction. No deconvolution was applied. d) Intensity line profiles as indicated in c). Note ~2x increase in peak intensity. e) Statistical comparison of relative peak intensity increase after AO correction at indicated depth in the sample. Data are derived from 10 beads. Means and standard deviations are shown. See also Fig. 2. Scale bars: 2 μm in a), 0.5 μm in c).
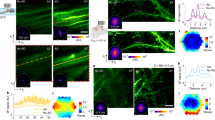
Supplementary Figure 6 AO improves 2P ISIM imaging at depth in Drosophila tissue.
a) Schematic of brain lobe, square indicates imaging region. Comparative images at indicated depth (rows), showing 2P excitation, no rescan (b); raw 2P ISIM (c); raw 2P ISIM with AO correction (d); 2P ISIM deconvolved after AO correction (e). (f) Wavefronts are shown before (left column) and after (right column) aberration correction; root mean square (RMS) values are also indicated. See also Fig. 1, Supplementary Video 1. Scale bars: 5 μm.
Supplementary Figure 7 2P ISIM images of Zygnema algae.
Before (a) and after (b) wavefront correction. Autofluorescence from the sample was used to derive wavefronts in each case, which are shown to the right of each image (RMS and peak-to-valley values are also shown). Images were acquired 7 μm into the stack (the largest algal cross-section in our imaging field) and ~50 μm from the coverslip. Scale bar: 5 μm.
Supplementary Figure 8 Fiducial-based AO Correction in live biological samples, as demonstrated in larval nematodes expressing GFP.
Neural structures were tagged with GFP in larval nematodes and imaged without rescan (a, no AO; d, with AO), with rescan (b, without AO; e, with AO) and with rescan and followed by deconvolution (c). The best resolution and contrast are obtained in (c). Lateral (upper) and axial maximum intensity projections are shown. The field of view was chosen near the head of the animal as indicated by red box in schematic f). Comparative wavefronts before and after AO correction are shown in (g); wavefronts were derived by scanning the beam over the neuron marked with red asterisk in (c). In this sample, the cell body region marked in (c) was used for AO. Scale bars: 5 μm.
Supplementary Figure 9 Fiducial-based AO correction improves 2P ISIM imaging of microtubule bundles in fixed cells embedded in a 3D matrix, at depths exceeding 200 μm from the coverslip surface.
Results without (a, d) and with (b, e) AO correction and subsequent deconvolution. The latter images show obvious improvements in spatial resolution and contrast (compare insets in a, b) and signal (line profiles in c, corresponding to red/green lines indicated in a, b. Representative images are shown 50 μm (a, b) and 230 μm (d, e) from the coverslip. From the MTFs (see Methods for details on calculations), we estimate that AO improves the lateral resolution from 0.45 μm (a) to 0.20 μm (b) and the axial resolution from 1.50 μm (a) to 0.55 μm (b) 50 μm from the coverslip. AO improved lateral resolution from 1.05 μm (d) to 0.33 μm (e) and axial resolution from 2.34 μm (d) to 0.65 μm (e) 230 μm from the coverslip. Images (d, e) also show the fluorescent bead (red asterisk in d) used for correction; note that image contrast has been adjusted to show the much fainter signal in the biological sample, which is why bead appears saturated. Comparative wavefronts corresponding to (d, e) are shown in (f). All images are maximum intensity projections. Scale bars: 5 μm.
Supplementary Figure 10 Dye-based AO improves 2P-ISIM imaging in somites (b-e) and neural tube (g-h) of live embryonic zebrafish at 30-32 hpf.
a) Schematic of embryo, indicating imaging region (higher magnification view). Selected imaging fields at indicated axial depth are shown, before (b, d, g) and after (c, e, h) AO correction and subsequent deconvolution. Comparative wavefronts are also shown as inset. f) Indicates spectral bandwidth used for AO correction with CellTracker Orange and 2P-ISIM imaging. See also Fig. 3. Scale bar: 10 μm.
Supplementary Figure 11 Dye-based AO improves 2P-ISIM imaging of gastrocnemius muscle of excised mouse leg.
Rhodamine phalloidin was used to stain actin fibers within the muscle leg and CellTracker Green was added for AO correction. a) Graph indicates spectral region used for AO correction vs. imaging. Selected image slices through two gastrocnemius muscle fibers of differing contractility before (b, e, h, k) and after (c, f, i, l) AO and deconvolution. Normalized line profiles (40 pixels in width) corresponding to red/black lines indicated in each depth are shown in (d, g, j). Higher magnification views of dashed yellow rectangular regions are shown, as are wavefronts (insets). Scale bars: 5 μm for zoomed-out panels, 2 μm for higher magnification views.
Supplementary Figure 12 Dye-based AO improves 2P-ISIM imaging of presumed gut granules in live larval C. elegans.
a) Schematic of larvae, indicating approximate region (higher magnification views) used in imaging. Worms were incubated overnight with CellTracker Orange mixed with OP50 prior to imaging. The 400 nm-700 nm spectral region was used for AO correction. Imaging was performed before (b, d, f, h, j) and after (c, e, g, i, k, all with subsequent deconvolution) AO, using a 525/70 bandpass emission filter. Scale bar in brightfield transmission image: 100 μm; in fluorescence images: 10 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–2. (PDF 2876 kb)
Supplementary Software
The software is compressed as a zip file. It includes two Python scripts that we used for the deconvolution of fluorescent beads, cells in gels, and zebrafish embryo datasets, three Matlab scripts for the deconvolution of the time-lapse volumetric embryo data, and a text file that describes how to use this software. (ZIP 23 kb)
2P-ISIM volume comparison (AO off, AO on + deconvolution) using labeled actin in Drosophila third instar larval brain
See also Fig. 1. (AVI 35799 kb)
AO 2P-ISIM enables volumetric time-lapse imaging of histone labeled GFP in nematode embryos
The aberrated wavefront was determined at a single plane in the middle of the stack, and was used for correction at all planes in the stack. Sixty imaging volumes were collected (imaging time for each stack: ~20 s, inter-stack interval: 40 s, total time interval between stacks: 1 minute), deconvolved, and the maximum intensity projections shown in this video. (AVI 3578 kb)
2P-ISIM volume comparison (AO off, AO on, AO on + deconvolution) using immunolabeled actin in fixed endothelial cells embedded in collagen matrices
Data were acquired 150 μm from the coverslip surface; projections were generated in ImageJ after isotropizing pixel size. See also Fig. 2. (AVI 62161 kb)
Rights and permissions
About this article
Cite this article
Zheng, W., Wu, Y., Winter, P. et al. Adaptive optics improves multiphoton super-resolution imaging. Nat Methods 14, 869–872 (2017). https://doi.org/10.1038/nmeth.4337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4337
This article is cited by
-
Live-cell imaging powered by computation
Nature Reviews Molecular Cell Biology (2024)
-
Deep tissue super-resolution imaging with adaptive optical two-photon multifocal structured illumination microscopy
PhotoniX (2023)
-
Label-free adaptive optics single-molecule localization microscopy for whole zebrafish
Nature Communications (2023)
-
Recent advances in optical imaging through deep tissue: imaging probes and techniques
Biomaterials Research (2022)
-
Multiple asters organize the yolk microtubule network during dclk2-GFP zebrafish epiboly
Scientific Reports (2022)