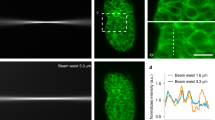
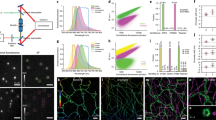
Abstract
The past quarter century has witnessed rapid developments of fluorescence microscopy techniques that enable structural and functional imaging of biological specimens at unprecedented depth and resolution. The performance of these methods in multicellular organisms, however, is degraded by sample-induced optical aberrations. Here I review recent work on incorporating adaptive optics, a technology originally applied in astronomical telescopes to combat atmospheric aberrations, to improve image quality of fluorescence microscopy for biological imaging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hooke, R. Micrographia: or some physiological descriptions of minute bodies made by magnifying glasses with observations and inquiries thereupon (Royal Society, 1665).
Schermelleh, L., Heintzmann, R. & Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 190, 165–175 (2010).
Power, R.M. & Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373 (2017).
Yang, W. & Yuste, R. In vivo imaging of neural activity. Nat. Methods 14, 349–359 (2017).
Gibson, S.F. & Lanni, F. Experimental test of an analytical model of aberration in an oil-immersion objective lens used in three-dimensional light microscopy. J. Opt. Soc. Am. A 9, 154–166 (1992).
Schwertner, M., Booth, M. & Wilson, T. Characterizing specimen induced aberrations for high NA adaptive optical microscopy. Opt. Express 12, 6540–6552 (2004).
Török, P., Hewlett, S.J. & Varga, P. The role of specimen-induced spherical aberration in confocal microscopy. J. Microsc. 188, 158–172 (1997).
Born, M. & Wolf, E. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light 7th edn. (Cambridge University Press, 1999).
Roorda,, A. in Wavefront Customized Visual Correction: The Quest for SuperVision II (eds. Macrae, S.M. et al.) Ch. 2 (Slack Inc., 2004).
Hartley, W.G. The light microscope: its use and development (Senecio Publishing Company, 1993).
Babcock, H.W. Adaptive optics revisited. Science 249, 253–257 (1990).
Hardy, J.W. Active optics: a new technology for the control of light. In Proc. IEEE 66, 651–697 (IEEE, 1978).
Booth, M.J. Adaptive optical microscopy: the ongoing quest for a perfect image. Light Sci. Appl. 3, e165 (2014).
Wang, K. et al. Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue. Nat. Commun. 6, 7276 (2015).
Liang, J., Williams, D.R. & Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 14, 2884–2892 (1997).
Azucena, O. et al. Wavefront aberration measurements and corrections through thick tissue using fluorescent microsphere reference beacons. Opt. Express 18, 17521–17532 (2010).
Azucena, O. et al. Adaptive optics wide-field microscopy using direct wavefront sensing. Opt. Lett. 36, 825–827 (2011).
Jorand, R. et al. Deep and clear optical imaging of thick inhomogeneous samples. PLoS One 7, e35795 (2012).
Rueckel, M., Mack-Bucher, J.A. & Denk, W. Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing. Proc. Natl. Acad. Sci. USA 103, 17137–17142 (2006).
Cha, J.W., Ballesta, J. & So, P.T. Shack-Hartmann wavefront-sensor-based adaptive optics system for multiphoton microscopy. J. Biomed. Opt. 15, 046022 (2010).
Aviles-Espinosa, R. et al. Measurement and correction of in vivo sample aberrations employing a nonlinear guide-star in two-photon excited fluorescence microscopy. Biomed. Opt. Express 2, 3135–3149 (2011).
Wang, K. et al. Rapid adaptive optical recovery of optimal resolution over large volumes. Nat. Methods 11, 625–628 (2014).
Tao, X. et al. Adaptive optics confocal microscopy using direct wavefront sensing. Opt. Lett. 36, 1062–1064 (2011).
Tao, X. et al. Adaptive optics microscopy with direct wavefront sensing using fluorescent protein guide stars. Opt. Lett. 36, 3389–3391 (2011).
Rahman, S.A. & Booth, M.J. Direct wavefront sensing in adaptive optical microscopy using backscattered light. Appl. Opt. 52, 5523–5532 (2013).
Tao, X. et al. Live imaging using adaptive optics with fluorescent protein guide-stars. Opt. Express 20, 15969–15982 (2012).
Ji, N., Milkie, D.E. & Betzig, E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2010).
Liu, R., Milkie, D.E., Kerlin, A., MacLennan, B. & Ji, N. Direct phase measurement in zonal wavefront reconstruction using multidither coherent optical adaptive technique. Opt. Express 22, 1619–1628 (2014).
Ji, N., Sato, T.R. & Betzig, E. Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex. Proc. Natl. Acad. Sci. USA 109, 22–27 (2012).
Wang, C. & Ji, N. Characterization and improvement of three-dimensional imaging performance of GRIN-lens-based two-photon fluorescence endomicroscopes with adaptive optics. Opt. Express 21, 27142–27154 (2013).
Wang, C. & Ji, N. Pupil-segmentation-based adaptive optical correction of a high-numerical-aperture gradient refractive index lens for two-photon fluorescence endoscopy. Opt. Lett. 37, 2001–2003 (2012).
Scrimgeour, J. & Curtis, J.E. Aberration correction in wide-field fluorescence microscopy by segmented-pupil image interferometry. Opt. Express 20, 14534–14541 (2012).
Daniel, E., Betzig, E. & Ji, N. Pupil-segmentation-based adaptive optical microscopy with full-pupil illumination. Opt. Lett. 36, 4206–4208 (2011).
Wang, C. et al. Multiplexed aberration measurement for deep tissue imaging in vivo. Nat. Methods 11, 1037–1040 (2014).
Gonsalves, R.A. Perspectives on phase retrieval and phase diversity in astronomy. In Proc. SPIE Vol. 9148 (eds. Marchetti, E. et al.) 91482P (SPIE, 2014).
Hanser, B.M., Gustafsson, M.G.L., Agard, D.A. & Sedat, J.W. Phase retrieval for high-numerical-aperture optical systems. Opt. Lett. 28, 801–803 (2003).
Hanser, B.M., Gustafsson, M.G.L., Agard, D.A. & Sedat, J.W. Phase-retrieved pupil functions in wide-field fluorescence microscopy. J. Microsc. 216, 32–48 (2004).
Kner, P., Winoto, L., Agard, D.A. & Sedat, J.W. Closed loop adaptive optics for microscopy without a wavefront sensor. In Proc. SPIE Vol. 7570 (eds. Conchello, J.-A. et al.) 7570 06 (SPIE, 2010).
Kner, P. Phase diversity for three-dimensional imaging. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 30, 1980–1987 (2013).
Albert, O., Sherman, L., Mourou, G., Norris, T.B. & Vdovin, G. Smart microscope: an adaptive optics learning system for aberration correction in multiphoton confocal microscopy. Opt. Lett. 25, 52–54 (2000).
Sherman, L., Ye, J.Y., Albert, O. & Norris, T.B. Adaptive correction of depth-induced aberrations in multiphoton scanning microscopy using a deformable mirror. J. Microsc. 206, 65–71 (2002).
Marsh, P., Burns, D. & Girkin, J. Practical implementation of adaptive optics in multiphoton microscopy. Opt. Express 11, 1123–1130 (2003).
Wright, A.J. et al. Exploration of the optimisation algorithms used in the implementation of adaptive optics in confocal and multiphoton microscopy. Microsc. Res. Tech. 67, 36–44 (2005).
Izeddin, I. et al. PSF shaping using adaptive optics for three-dimensional single-molecule super-resolution imaging and tracking. Opt. Express 20, 4957–4967 (2012).
Skorsetz, M., Artal, P. & Bueno, J.M. Performance evaluation of a sensorless adaptive optics multiphoton microscope. J. Microsc. 261, 249–258 (2016).
Galwaduge, P.T., Kim, S.H., Grosberg, L.E. & Hillman, E.M.C. Simple wavefront correction framework for two-photon microscopy of in vivo brain. Biomed. Opt. Express 6, 2997–3013 (2015).
Booth, M. Wave front sensor-less adaptive optics: a model-based approach using sphere packings. Opt. Express 14, 1339–1352 (2006).
Booth, M.J., Neil, M.A.A., Juskaitis, R. & Wilson, T. Adaptive aberration correction in a confocal microscope. Proc. Natl. Acad. Sci. USA 99, 5788–5792 (2002).
Booth, M.J. Wavefront sensorless adaptive optics for large aberrations. Opt. Lett. 32, 5–7 (2007).
Débarre, D., Botcherby, E.J., Booth, M.J. & Wilson, T. Adaptive optics for structured illumination microscopy. Opt. Express 16, 9290–9305 (2008).
Débarre, D. et al. Image-based adaptive optics for two-photon microscopy. Opt. Lett. 34, 2495–2497 (2009).
Bourgenot, C., Saunter, C.D., Taylor, J.M., Girkin, J.M. & Love, G.D. 3D adaptive optics in a light sheet microscope. Opt. Express 20, 13252–13261 (2012).
Booth, M., Andrade, D., Burke, D., Patton, B. & Zurauskas, M. Aberrations and adaptive optics in super-resolution microscopy. Microscopy (Oxf.) 64, 251–261 (2015).
Burke, D., Patton, B., Huang, F., Bewersdorf, J. & Booth, M.J. Adaptive optics correction of specimen-induced aberrations in single-molecule switching microscopy. Optica 2, 177–185 (2015).
Huang, F. et al. Ultra-high resolution 3D imaging of whole cells. Cell 166, 1028–1040 (2016).
Tehrani, K.F., Xu, J., Zhang, Y., Shen, P. & Kner, P. Adaptive optics stochastic optical reconstruction microscopy (AO-STORM) using a genetic algorithm. Opt. Express 23, 13677–13692 (2015).
McGorty, R., Schnitzbauer, J., Zhang, W. & Huang, B. Correction of depth-dependent aberrations in 3D single-molecule localization and super-resolution microscopy. Opt. Lett. 39, 275–278 (2014).
von Diezmann, A., Lee, M.Y., Lew, M.D. & Moerner, W.E. Correcting field-dependent aberrations with nanoscale accuracy in three-dimensional single-molecule localization microscopy. Optica 2, 985–993 (2015).
Arigovindan, M., Sedat, J.W. & Agard, D.A. Effect of depth dependent spherical aberrations in 3D structured illumination microscopy. Opt. Express 20, 6527–6541 (2012).
Thomas, B., Wolstenholme, A., Chaudhari, S.N., Kipreos, E.T. & Kner, P. Enhanced resolution through thick tissue with structured illumination and adaptive optics. J. Biomed. Opt. 20, 26006 (2015).
Auksorius, E. et al. Stimulated emission depletion microscopy with a supercontinuum source and fluorescence lifetime imaging. Opt. Lett. 33, 113–115 (2008).
Lenz, M.O. et al. 3-D stimulated emission depletion microscopy with programmable aberration correction. J. Biophotonics 7, 29–36 (2014).
Gould, T.J., Burke, D., Bewersdorf, J. & Booth, M.J. Adaptive optics enables 3D STED microscopy in aberrating specimens. Opt. Express 20, 20998–21009 (2012).
Mosk, A.P., Lagendijk, A., Lerosey, G. & Fink, M. Controlling waves in space and time for imaging and focusing in complex media. Nat. Photonics 6, 283–292 (2012).
Horstmeyer, R., Ruan, H. & Yang, C. Guidestar-assisted wavefront-shaping methods for focusing light into biological tissue. Nat. Photonics 9, 563–571 (2015).
Vellekoop, I.M. Feedback-based wavefront shaping. Opt. Express 23, 12189–12206 (2015).
Sinefeld, D., Paudel, H.P., Ouzounov, D.G., Bifano, T.G. & Xu, C. Adaptive optics in multiphoton microscopy: comparison of two, three and four photon fluorescence. Opt. Express 23, 31472–31483 (2015).
Jesacher, A. et al. Adaptive harmonic generation microscopy of mammalian embryos. Opt. Lett. 34, 3154–3156 (2009).
Sun, W., Tan, Z., Mensh, B.D. & Ji, N. xThalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315 (2016).
Acknowledgements
The author thanks A. Roorda and R. Turcotte for providing data in Figure 2. This work is supported by Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Ji, N. Adaptive optical fluorescence microscopy. Nat Methods 14, 374–380 (2017). https://doi.org/10.1038/nmeth.4218
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4218
This article is cited by
-
Live-cell imaging powered by computation
Nature Reviews Molecular Cell Biology (2024)
-
Vascularized organoid-on-a-chip: design, imaging, and analysis
Angiogenesis (2024)
-
Vectorial adaptive optics
eLight (2023)
-
Tracing multiple scattering trajectories for deep optical imaging in scattering media
Nature Communications (2023)
-
Universal adaptive optics for microscopy through embedded neural network control
Light: Science & Applications (2023)