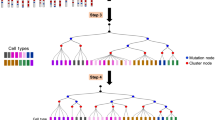
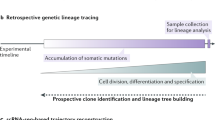
Abstract
We present an approach for engineering evolving DNA barcodes in living cells. A homing guide RNA (hgRNA) scaffold directs the Cas9–hgRNA complex to the DNA locus of the hgRNA itself. We show that this homing CRISPR–Cas9 system acts as an expressed genetic barcode that diversifies its sequence and that the rate of diversification can be controlled in cultured cells. We further evaluate these barcodes in cell populations and show that they can be used to record lineage history and that the barcode RNA can be amplified in situ, a prerequisite for in situ sequencing. This integrated approach will have wide-ranging applications, such as in deep lineage tracing, cellular barcoding, molecular recording, dissecting cancer biology, and connectome mapping.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Sulston, J.E., Schierenberg, E., White, J.G. & Thomson, J.N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 (1983).
Kretzschmar, K. & Watt, F.M. Lineage tracing. Cell 148, 33–45 (2012).
Weisblat, D.A., Sawyer, R.T. & Stent, G.S. Cell lineage analysis by intracellular injection of a tracer enzyme. Science 202, 1295–1298 (1978).
Dymecki, S.M. & Tomasiewicz, H. Using Flp-recombinase to characterize expansion of Wnt1-expressing neural progenitors in the mouse. Dev. Biol. 201, 57–65 (1998).
Walsh, C. & Cepko, C.L. Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science 255, 434–440 (1992).
Porter, S.N., Baker, L.C., Mittelman, D. & Porteus, M.H. Lentiviral and targeted cellular barcoding reveals ongoing clonal dynamics of cell lines in vitro and in vivo. Genome Biol. 15, R75 (2014).
Lu, R., Neff, N.F., Quake, S.R. & Weissman, I.L. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat. Biotechnol. 29, 928–933 (2011).
Mali, P., Esvelt, K.M. & Church, G.M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963 (2013).
Lee, J.H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014).
Church, G.M., Marblestone, A.H. & Kalhor, R. in The Future of the Brain: Essays by the World's Leading Neuroscientists (eds. Marcus, G. & Freeman, J.) 50–66 (Princeton University Press, 2016).
Peikon, I.D., Gizatullina, D.I. & Zador, A.M. In vivo generation of DNA sequence diversity for cellular barcoding. Nucleic Acids Res. 42, e127 (2014).
Naik, S.H., Schumacher, T.N. & Perié, L. Cellular barcoding: a technical appraisal. Exp. Hematol. 42, 598–608 (2014).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Malina, A. et al. PAM multiplicity marks genomic target sites as inhibitory to CRISPR-Cas9 editing. Nat. Commun. 6, 10124 (2015).
Ke, R. et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat. Methods 10, 857–860 (2013).
Lee, J.H. et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat. Protoc. 10, 442–458 (2015).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517, 583–588 (2015).
Liu, Z. & Keller, P.J. Emerging imaging and genomic tools for developmental systems biology. Dev. Cell 36, 597–610 (2016).
Junker, J.P. et al. Massively parallel whole-organism lineage tracing using CRISPR/Cas9 induced genetic scars. Preprint at bioRxiv http://dx.doi.org/10.1101/056499 (2016).
McKenna, A. et al. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907 (2016).
Perli, S.D., Cui, C.H. & Lu, T.K. Continuous genetic recording with self-targeting CRISPR-Cas in human cells. Science 353, aag0511 (2016).
Marblestone, A.H. et al. Physical principles for scalable neural recording. Front. Comput. Neurosci. 7, 137 (2013).
Marblestone, A.H. et al. Conneconomics: The Economics of Dense, Large-Scale, High-Resolution Neural Connectomics. Preprint at bioRxiv http://dx.doi.org/10.1101/001214 (2013).
Platt, R.J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Certo, M.T. et al. Tracking genome engineering outcome at individual DNA breakpoints. Nat. Methods 8, 671–676 (2011).
Komor, A.C., Kim, Y.B., Packer, M.S., Zuris, J.A. & Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Yang, L. et al. Optimization of scarless human stem cell genome editing. Nucleic Acids Res. 41, 9049–9061 (2013).
Schirmer, M. et al. Insight into biases and sequencing errors for amplicon sequencing with the Illumina MiSeq platform. Nucleic Acids Res. 43, e37 (2015).
Becker, R.A., Chambers, J.M. & Wilks, A.R. The New S Language: A Programming Environment for Data Analysis and Graphics (Chapman & Hall/CRC, 1988).
Acknowledgements
The authors would like to acknowledge W.L. Chew, J. Aach, S. Byrne, E. Daugharthy, T. Ferrante, J.H. Lee, M. Moosburner, I. Peikon, H. Lee, A. Ng, J. Fernandez Juarez, A. Marblestone, A. Chavez, Y. Mayshar, J. Scheiman, K. Kalhor, T. Wu, J. Shendure, and T. Lu for helpful comments or discussions and the Biopolymers Facility at HMS for technical assistance. This work has been supported by funding from NIH grants MH103910 and HG005550 (G.M.C.) and the Intelligence Advanced Research Projects Activity (IARPA) via Department of Interior/Interior Business Center (DoI/IBC) contract number D16PC00008 (G.M.C.), and by UCSD new faculty startup funds (P.M.).
Author information
Authors and Affiliations
Contributions
R.K., P.M., and G.M.C. conceived the study. R.K. and P.M. carried out the experiments. R.K. analyzed the data. R.K. and P.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.K., P.M. and G.M.C. have filed provisional patent applications based on this study.
Integrated supplementary information
Supplementary Figure 1 Comparing canonical and homing guide RNAs.
(a) Primary sequences and the predicted secondary structures of canonical and homing gRNAs. Positions that were mutated to derive the hgRNA are underlined. Orange bases mark the newly created PAM in hgRNA. Grey boxes mark all the positions where hgRNA is mutated compared to canonical sgRNA. (b) Results of a HR-based assay to evaluate to functionality of hgRNAs. A genomically integrated GFP coding sequence is disrupted by the insertion of a stop codon and a 68-bp genomic fragment from the AAVS1 locus. Restoration of the GFP sequence by HR with an appropriate donor sequence results in GFP+ cells that can be quantified by FACS. AAVS1 locus contains a site known as T1 which matches the spacer sequence of hgRNA-A21. Bar graph on top depicts HR efficiencies induced by wild type and homing T1 guide RNAs, as measured by FACS. Representative FACS plots of the targeted cells are depicted below. Data are shown as means ± s.e.m (n=4). (c) Sequencing results showing above-background accumulation of mutations in hgRNA locus upon Cas9 expression. Cas9 expression is induced in cells with a lentivirally integrated hgRNA-A21. DNA samples harvested before (t=0 days) and at various points after induction are characterized by high-throughput sequencing to quantify mutations in the DNA locus that encodes hgRNA-A21. Any mismatch, deletion, or insertions compared to the original locus is considered a mutation. The high initial fraction of mutants is due to sequencing error (materials and methods) as the steady level of mutations in the non-induced sample suggests there is no significant Cas9 expression leakage in the course of our experimental times. (d) Comparison of viability between cells containing hg and sgRNAs. Cells containing an sgRNA were mixed with cells containing an hgRNA with the same spacer sequence on day 0. Both cells also included the target site for the sg and hgRNA. Cas9 expression was induced and samples were taken at various points after induction up to 20 days. Sequencing was then used to identify the fraction of cell at each point with each gRNA as represented by the fraction of reads that match each kind of scaffold (Methods). The results show a similar viability for cells carrying sgRNAs and hgRNAs.
Supplementary Figure 2 Longer variants of homing gRNAs.
Design of four longer variants of hgRNA-A21 is shown. Stuffer sequences of 5, 30, 55, or 80 base pairs were added upstream of a spacer very similar to the A21 spacer to obtain the four increasingly lengthy A’ variants. (B,C,D) Cas9 is induced in cell lines with the hgRNAs from panel A integrated lentivirally. All samples were harvested in the end; however, induction was initiated at 1, 2, 3, or 4 weeks before harvest with one sample was not being induced (0 weeks of induction). DNA was extracted from all samples and characterized by high-throughput sequencing to quantify mutations and functionality of hgRNA loci. Any mismatch, deletion, or insertion compared to the original locus is considered a mutation. The high pre-induction fraction of mutants is likely due to a combination of sequencing error (materials and methods) and background Cas9 expression leakage which becomes a significant factor during the 4-week course of the experiment. The drop in total diversity of hgRNA-A21 after the second week of induction is likely due to loss of diversity due to the bottle-necking effect of passaging the cells.
Supplementary Figure 3 Target-specific in situ detection of gRNA molecules expressed by RNA polymerase III.
(a) A schematic of our in situ amplification and detection assay based on FISSEQ. (b) Results of target-specific in situ amplification and detection for two different hgRNA constructs and a negative control. The schematic on top shows the position of the reverse-transcription (RT) primer in each design. The bottom panels show a representative field of view from each of two biological replicates. Amplicons are labeled with Cy5 (orange) and nuclei are labeled with DAPI (blue).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1 and 2, and Supplementary Note (PDF 1878 kb)
Source data
Rights and permissions
About this article
Cite this article
Kalhor, R., Mali, P. & Church, G. Rapidly evolving homing CRISPR barcodes. Nat Methods 14, 195–200 (2017). https://doi.org/10.1038/nmeth.4108
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4108
This article is cited by
-
Bone serves as a transfer station for secondary dissemination of breast cancer
Bone Research (2023)
-
Dissecting metastasis using preclinical models and methods
Nature Reviews Cancer (2023)
-
Clonal tracking in cancer and metastasis
Cancer and Metastasis Reviews (2023)
-
Clonal relations in the mouse brain revealed by single-cell and spatial transcriptomics
Nature Neuroscience (2022)
-
Massively parallel genomic perturbations with multi-target CRISPR interrogates Cas9 activity and DNA repair at endogenous sites
Nature Cell Biology (2022)