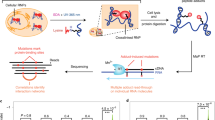
Abstract
Protein-RNA networks are ubiquitous and central in biological control. We present an approach termed RNA Tagging that enables the user to identify protein-RNA interactions in vivo by analyzing purified cellular RNA, without protein purification or cross-linking. An RNA-binding protein of interest is fused to an enzyme that adds uridines to the end of RNA. RNA targets bound by the chimeric protein in vivo are covalently marked with uridines and subsequently identified from extracted RNA via high-throughput sequencing. We used this approach to identify hundreds of RNAs bound by a Saccharomyces cerevisiae PUF protein, Puf3p. The results showed that although RNA-binding proteins productively bind specific RNAs to control their function, they also 'sample' RNAs without exerting a regulatory effect. We used the method to uncover hundreds of new and likely regulated targets for a protein without canonical RNA-binding domains, Bfr1p. RNA Tagging is well suited to detect and analyze protein-RNA networks in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
McHugh, C.A., Russell, P. & Guttman, M. Methods for comprehensive experimental identification of RNA-protein interactions. Genome Biol. 15, 203 (2014).
Tenenbaum, S.A., Carson, C.C., Lager, P.J. & Keene, J.D. Identifying mRNA subsets in messenger ribonucleoprotein complexes by using cDNA arrays. Proc. Natl. Acad. Sci. USA 97, 14085–14090 (2000).
Zhao, J. et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell 40, 939–953 (2010).
Ule, J. et al. CLIP identifies Nova-regulated RNA networks in the brain. Science 302, 1212–1215 (2003).
Licatalosi, D.D. et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 456, 464–469 (2008).
Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141 (2010).
König, J. et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 17, 909–915 (2010).
Mili, S. & Steitz, J.A. Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA 10, 1692–1694 (2004).
Riley, K.J., Yario, T.A. & Steitz, J.A. Association of Argonaute proteins and microRNAs can occur after cell lysis. RNA 18, 1581–1585 (2012).
Riley, K.J. & Steitz, J.A. The “Observer Effect” in genome-wide surveys of protein-RNA interactions. Mol. Cell 49, 601–604 (2013).
Darnell, R.B. HITS-CLIP: panoramic views of protein-RNA regulation in living cells. Wiley Interdiscip. Rev. RNA 1, 266–286 (2010).
Fecko, C.J. et al. Comparison of femtosecond laser and continuous wave UV sources for protein-nucleic acid crosslinking. Photochem. Photobiol. 83, 1394–1404 (2007).
Lapointe, C.P. & Wickens, M. The nucleic acid-binding domain and translational repression activity of a Xenopus terminal uridylyl transferase. J. Biol. Chem. 288, 20723–20733 (2013).
Kim, B. et al. TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. EMBO J. 34, 1801–1815 (2015).
Gerber, A.P., Herschlag, D. & Brown, P.O. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2, E79 (2004).
Zhu, D., Stumpf, C.R., Krahn, J.M., Wickens, M. & Hall, T.M. A 5′ cytosine binding pocket in Puf3p specifies regulation of mitochondrial mRNAs. Proc. Natl. Acad. Sci. USA 106, 20192–20197 (2009).
Olivas, W. & Parker, R. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 19, 6602–6611 (2000).
Saint-Georges, Y. et al. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS One 3, e2293 (2008).
Gadir, N., Haim-Vilmovsky, L., Kraut-Cohen, J. & Gerst, J.E. Localization of mRNAs coding for mitochondrial proteins in the yeast Saccharomyces cerevisiae. RNA 17, 1551–1565 (2011).
Chatenay-Lapointe, M. & Shadel, G.S. Repression of mitochondrial translation, respiration and a metabolic cycle-regulated gene, SLF1, by the yeast Pumilio-family protein Puf3p. PLoS One 6, e20441 (2011).
García-Rodriguez, L.J., Gay, A.C. & Pon, L.A. Puf3p, a Pumilio family RNA binding protein, localizes to mitochondria and regulates mitochondrial biogenesis and motility in budding yeast. J. Cell Biol. 176, 197–207 (2007).
Wilinski, D. et al. RNA regulatory networks diversified through curvature of the PUF protein scaffold. Nat. Commun. 6, 8213 (2015).
Kusov, Y.Y., Shatirishvili, G., Dzagurov, G. & Gauss-Muller, V. A new G-tailing method for the determination of the poly(A) tail length applied to hepatitis A virus RNA. Nucleic Acids Res. 29, E57-7 (2001).
Lane, A.N., Chaires, J.B., Gray, R.D. & Trent, J.O. Stability and kinetics of G-quadruplex structures. Nucleic Acids Res. 36, 5482–5515 (2008).
Freeberg, M.A. et al. Pervasive and dynamic protein binding sites of the mRNA transcriptome in Saccharomyces cerevisiae. Genome Biol. 14, R13 (2013).
Bailey, T.L. & Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2, 28–36 (1994).
Miller, M.A., Russo, J., Fischer, A.D., Lopez Leban, F.A. & Olivas, W.M. Carbon source-dependent alteration of Puf3p activity mediates rapid changes in the stabilities of mRNAs involved in mitochondrial function. Nucleic Acids Res. 42, 3954–3970 (2014).
Wickens, M., Bernstein, D.S., Kimble, J. & Parker, R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 18, 150–157 (2002).
Hogan, D.J., Riordan, D.P., Gerber, A.P., Herschlag, D. & Brown, P.O. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 6, e255 (2008).
Jackson, J.S. Jr., Houshmandi, S.S., Lopez Leban, F. & Olivas, W.M. Recruitment of the Puf3 protein to its mRNA target for regulation of mRNA decay in yeast. RNA 10, 1625–1636 (2004).
Houshmandi, S.S. & Olivas, W.M. Yeast Puf3 mutants reveal the complexity of Puf-RNA binding and identify a loop required for regulation of mRNA decay. RNA 11, 1655–1666 (2005).
Huh, W.K. et al. Global analysis of protein localization in budding yeast. Nature 425, 686–691 (2003).
Williams, C.C., Jan, C.H. & Weissman, J.S. Targeting and plasticity of mitochondrial proteins revealed by proximity-specific ribosome profiling. Science 346, 748–751 (2014).
Sun, M. et al. Global analysis of eukaryotic mRNA degradation reveals Xrn1-dependent buffering of transcript levels. Mol. Cell 52, 52–62 (2013).
Jackson, C.L. & Kepes, F. BFR1, a multicopy suppressor of brefeldin A-induced lethality, is implicated in secretion and nuclear segregation in Saccharomyces cerevisiae. Genetics 137, 423–437 (1994).
Trautwein, M., Dengjel, J., Schirle, M. & Spang, A. Arf1p provides an unexpected link between COPI vesicles and mRNA in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 5021–5037 (2004).
Lang, B.D., Li, A., Black-Brewster, H.D. & Fridovich-Keil, J.L. The brefeldin A resistance protein Bfr1p is a component of polyribosome-associated mRNP complexes in yeast. Nucleic Acids Res. 29, 2567–2574 (2001).
Weidner, J., Wang, C., Prescianotto-Baschong, C., Estrada, A.F. & Spang, A. The polysome-associated proteins Scp160 and Bfr1 prevent P body formation under normal growth conditions. J. Cell Sci. 127, 1992–2004 (2014).
Simpson, C.E., Lui, J., Kershaw, C.J., Sims, P.F. & Ashe, M.P. mRNA localization to P-bodies in yeast is bi-phasic with many mRNAs captured in a late Bfr1p-dependent wave. J. Cell Sci. 127, 1254–1262 (2014).
Ast, T., Cohen, G. & Schuldiner, M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell 152, 1134–1145 (2013).
Mitchell, S.F., Jain, S., She, M. & Parker, R. Global analysis of yeast mRNPs. Nat. Struct. Mol. Biol. 20, 127–133 (2013).
Jan, C.H., Williams, C.C. & Weissman, J.S. Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science 346, 1257521 (2014).
Munoz-Tello, P., Rajappa, L., Coquille, S. & Thore, S. Polyuridylation in eukaryotes: a 3′-end modification regulating RNA life. Biomed. Res. Int. 2015, 968127 (2015).
Norbury, C.J. Cytoplasmic RNA: a case of the tail wagging the dog. Nat. Rev. Mol. Cell Biol. 14, 643–653 (2013).
Chang, H., Lim, J., Ha, M. & Kim, V.N. TAIL-seq: genome-wide determination of poly(A) tail length and 3′ end modifications. Mol. Cell 53, 1044–1052 (2014).
Newman, M.A., Mani, V. & Hammond, S.M. Deep sequencing of microRNA precursors reveals extensive 3′ end modification. RNA 17, 1795–1803 (2011).
Jackson, R.J., Hellen, C.U. & Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Miller, J.E. & Reese, J.C. Ccr4-Not complex: the control freak of eukaryotic cells. Crit. Rev. Biochem. Mol. Biol. 47, 315–333 (2012).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Hulsen, T., de Vlieg, J. & Alkema, W. BioVenn—a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics 9, 488 (2008).
Xu, Z. et al. Bidirectional promoters generate pervasive transcription in yeast. Nature 457, 1033–1037 (2009).
Crooks, G.E., Hon, G., Chandonia, J.M. & Brenner, S.E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 (2001).
Acknowledgements
We thank members of the Wickens lab for helpful comments and suggestions throughout the work, and for their thoughtful discussions of the manuscript. We appreciate discussions with S. Kennedy (Harvard University), P. Anderson (University of Wisconsin–Madison) and their labs, as well as discussions and efforts with E. Grayhack and E. Phizicky (University of Rochester) in early stages of the work. We thank J. Kimble and E. Sorokin (University of Wisconsin–Madison) for use of a computational server, and L. Vanderploeg of the Biochemistry Media Lab for help with the figures. We also thank the University of Wisconsin Biotechnology Center DNA Sequencing Facility, particularly M. Adams and M. Sussman, for high-throughput sequencing facilities and services. The work was supported by the US National Institutes of Health (grant GM50942) and by Wharton and Biochemistry Scholar Fellowships to C.P.L.
Author information
Authors and Affiliations
Contributions
C.P.L. and M.W. conceived of the method and designed the experiments. C.P.L. performed the experiments. C.P.L., D.W., H.A.J.S. and M.W. analyzed the data. C.P.L. and M.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.P.L. and M.W. have filed a patent that encompasses the RNA Tagging approach.
Integrated supplementary information
Supplementary Figure 1 RNA Tagging identified in vivo protein-RNA interactions.
a) Schematic of the RT-PCR assay for targeted RNA Tagging. The RT primers and PCR primer sets used in panels b and c are shown. PCR primer set 1 was two gene-specific primers and primer set 2 used a gene-specific forward primer and the U-select RT primer as the reverse PCR primer. (b,c) PUF3-PUP tagged HSP10 (b) and COX17 (c) mRNAs. In each panel, gel slices were run on the same gel and were separated here for clarity. RT and PCR primers used in each column are indicated. “-RT” lanes (no reverse transcriptase) monitored genomic DNA contamination, which was minimal. “dT” lanes used the oligo(dT) primer, and illustrate that polyadenylated mRNA was present in all samples. “U-sel” lanes used the U-select primer, which detects RNAs with U-tags. The control strain (BY4742) lacked an RNA Tagging chimera. PUF3-PUP is the active RNA Tagging chimera and PUF3-PUPmut is a catalytically inactive chimera, which harbors active site mutations in the PUP (Asp185Ala, Asp187Ala). d) Representative Sanger sequencing results of tagged HSP10 mRNA. The PCR product from the U-select (U-sel) lane of the PUF3-PUP sample in panel b was cloned and individual colonies were sequenced. Black text indicates genomically encoded HSP10 3ʹ UTR sequence, bold blue text indicates non-genomically encoded adenosines (the poly(A) tail), and bold red text indicates non-genomically encoded thymidines, which represent the 3ʹ U-tag added by PUF3-PUP. e) PHD1 mutant alleles. The two PUF-binding elements in PHD1 mRNA were disrupted via UGU to ACA substitutions in the endogenous PHD1 locus. Active or inactive (DD185/187AA) versions of PUP-2 were fused to the endogenous copy of PUF5 (PUF5-PUP and PUF5-PUPmutant, respectively) in the wild-type and mutant PHD1 strains. f) PUF5-PUP requires its binding elements to tag PHD1 mRNA. Lanes are as in panels b and c.
Supplementary Figure 2 Illumina sequencing accurately detected U-tags of multiple lengths.
a) Synthetic libraries with various length U-tags, shown here as the reverse complement for clarity. The indicated libraries were paired-end sequenced on an Illumina HiSeq2500. The purple sequence represents the Illumina 5ʹ adapter, the blue sequence represents a poly(A) tail of 12 nucleotides, the red sequence represents U-tags of multiple lengths, and the black sequence represents the U-select RT primer. The starred (*) position in the U0 library was further analyzed in panel b. b) Accuracy of identifying Tagged RNAs by a single non-templated uridine. The percent nucleotide composition of position 13 in Read 2 of the U0 library, which corresponds to the starred (*) position in panel a, was calculated and plotted (n = 310,745). The actual bases detected by sequencing were reverse complemented here for clarity.
Supplementary Figure 3 Comparison of Puf3p RNA Tagging results and RNA abundance.
a) The mean number of Tagged RNAs detected for Puf3p targets was correlated with the mean length of their U-tag (ρ = 0.5, P = 0, n = 476). Spearman’s correlation coefficient (ρ) and associated P-value (P) are indicated. TRPM, Tagged RNAs Per Million uniquely mapped reads. b) The mean number of Tagged RNAs (TRPM) detected for Puf3p targets was uncorrelated with their mean abundance (Spearman correlation, P > 0.1). FPKM, fragments per kilobase of exon per million reads mapped. c) The mean length of the U-tag on Puf3p targets was weakly correlated with their mean abundance (FPKM) (ρ = −0.37, P = 0, n = 476). Spearman’s correlation coefficient (ρ) and associated P-value (P) are indicated.
Supplementary Figure 4 Comparison of Puf3p-binding elements identified with multiple methods.
Proportional Venn diagram of Puf3p targets identified using RNA Tagging, RIP-chip15, and PAR-CLIP25. The numbers indicate the number targets in each area of the plot. Position-weight matrices (plotted in bits) of the Puf3p-binding elements (PBEs) found in each group of targets are indicated. PBEs were derived as follows: PBEs with grey stars, MEME analysis of all RNA Tagging targets; PBEs with grey squares, MEME analysis of all RIP-chip targets; PBEs with grey triangles, PBEs in PAR-CLIP peaks.
Supplementary Figure 5 Puf3p target rank was correlated with TRPM and U-tag length but was largely uncorrelated with RNA abundance.
a) The mean number of Tagged RNAs (TRPM) detected for Puf3p targets was correlated with their RNA Tagging rank (ρ = −0.91, P = 0, n = 476). Spearman’s correlation coefficients (ρ) and associated P-values (P) are indicated in all panels. TRPM, Tagged RNAs Per Million uniquely mapped reads. b) The RNA Tagging rank of Puf3p targets was correlated with the mean length of their U-tags (ρ = −0.75, P = 0, n = 476). c) RNA Tagging rank of Puf3p targets was largely uncorrelated with their mean RNA abundance (ρ = 0.16, P = 0.0007, n = 476). FPKM, fragments per kilobase of exon per million reads mapped.
Supplementary Figure 6 Comparison of RNA abundance and the position of binding elements across Puf3p targets.
a) Class C targets were the most abundant Puf3p targets. Empirical cumulative distributions of RNA abundance were plotted for all Puf3p targets (left) and the three Puf3p target classes (middle) relative to all mRNAs (all mRNAs, n = 6,595; Class A, n = 92; Class B, n = 189; Class C, n = 195). The P-values from Kolmogorov-Smirnov (KS) tests comparing the different distributions are indicated (right). b,c) Puf3p-binding elements were similarly positioned in the 3ʹ UTRs of each class of Puf3p targets. The distance from each binding element to the 3ʹ terminus (b) and the stop codon (c) of the target was calculated and plotted (all targets, n = 404; Class A, n = 90; Class B, n = 169; Class C, n = 145) (Tukey whiskers indicated). There were no statistical differences between any of the groups (Fisher-Pitman permutation tests, P > 0.1). d,e) The mean number of Tagged RNAs (d) and number of U’s (e) detected for targets were compared to the distance from the PBE to the 3ʹ terminus for isoforms of 64 Puf3p targets (143 distinct mRNAs) detected by at least 31 reads (24,417 reads total). No significant correlations were observed (Pearson and Spearman correlations, P > 0.1).
Supplementary Figure 7 The number of tagged RNAs and U-tag length were correlated with in vitro binding affinity.
a) Published in vitro binding affinity data of purified Puf3p for the six indicated RNA sequences was obtained and shown here16. b,c) The median number of Tagged RNAs detected (TRPM) (r= −0.97, P = 0.0013; ρ= −0.94, P = 0.005; n = 6) (b) and median U-tag length (r= −0.94, P = 0.0051; ρ= −1, P = 0; n = 6) (c) of Puf3p targets containing six distinct binding elements was calculated and compared to the published in vitro binding affinity (Kd) of purified Puf3p for those sequences. Pearson’s (r) and Spearman’s (ρ) correlation coefficients and the associated P-values (P) are indicated. TRPM, Tagged RNAs Per Million uniquely mapped reads.
Supplementary Figure 8 Puf3p targets were enriched for mRNAs translated at mitochondria in the absence of cycloheximide.
Published mitochondria-specific ribosome profiling (RP) data in the absence of cycloheximide was mined33. Empirical cumulative distributions were plotted for all Puf3p targets (left) and the Puf3p target classes (middle) relative to all mRNAs (all mRNAs, n = 5,609; Class A, n = 92; Class B, n = 188; Class C, n = 193). The P-values from Kolmogorov-Smirnov (KS) tests that compared the different distributions are indicated (right).
Supplementary Figure 9 mRNAs with known PUF3-dependent half-lives were class A or class B targets.
Summary of published RNA half-lives of the indicated genes in wild-type and puf3Δ strains27. Puf3p target class, RNA Tagging rank, and Puf3p-binding elements are indicated. “NA” indicates the gene was not identified as a Puf3p target by RNA Tagging.
Supplementary Figure 10 Comparison of Bfr1p RNA Tagging results and RNA abundance.
a) The mean number of Tagged RNAs (TRPM) detected and the mean length of their U-tag were uncorrelated (Spearman correlation, P > 0.1). TRPM, Tagged RNAs Per Million uniquely mapped reads. b) The mean number of Tagged RNAs (TRPM) detected for Bfr1p targets was weakly correlated with their mean abundance (ρ= 0.3, P = 0; n = 1,298). FPKM, fragments per kilobase of exon per million reads mapped. Spearman’s correlation coefficient (ρ) and associated P-value (P) are indicated. c) The mean length of the U-tag on Bfr1p targets was largely uncorrelated with their mean abundance (ρ= −0.12, P = 0; n = 1,298). Spearman’s correlation coefficient (ρ) and associated P-value (P) are indicated.
Supplementary Figure 11 Bfr1p targets identified by both RNA Tagging and RIP-chip were enriched for membrane-associated functions.
Proportional Venn diagram of Bfr1p targets identified using RNA Tagging and RIP-chip29. GO analyses were performed on the three groups and enrichments for representative terms from Biological Process and Cellular Component ontologies are indicated (see Supplementary Data 2 for complete lists).
Supplementary Figure 12 Bfr1p target rank was correlated with TRPM and was very weakly correlated with U-tag length and RNA abundance.
a) The mean number of Tagged RNAs (TRPM) detected for Bfr1p targets was correlated with their RNA Tagging rank (ρ= −0.87, P = 0; n = 1,298). Spearman’s correlation coefficients (ρ) and associated P-values (P) are indicated in all panels. TRPM, Tagged RNAs Per Million uniquely mapped reads. b) The RNA Tagging rank of Bfr1p targets was weakly correlated with the mean length of their U-tags (ρ= −0.35, P = 0; n = 1,298). c) RNA Tagging rank of Bfr1p targets was weakly correlated with their mean RNA abundance (ρ= −0.28, P = 0; n = 1,298). FPKM, fragments per kilobase of exon per million reads mapped.
Supplementary Figure 13 Bfr1p target class was correlated with protein localization to the ER.
The fraction of each class of Bfr1p targets that are localized to the cytoplasm, endoplasmic reticulum (ER), nucleus, mitochondria, and nucleolus, obtained from the yeast GFP database32, was plotted. Classes A-C of Bfr1p targets were highly enriched for ER-localized proteins (hypergeometric tests, P < 1 x10−16), and the enrichment progressively decreased from Class A to D targets. No other significant enrichments were observed (hypergeometric tests, P > 0.01).
Supplementary Figure 14 Bfr1p targets were highly enriched for abundant mRNAs.
Empirical cumulative distributions of RNA abundance (FPKM) were plotted for all Bfr1p targets (left) and the Bfr1p target classes (middle) relative to all mRNAs (all mRNAs, n = 6,595; Class A, n = 174; Class B, n = 297; Class C, n = 564; Class D, n = 261). The P-values from Kolmogorov-Smirnov (KS) tests comparing the different distributions are indicated (right). Class A Bfr1p targets were most enriched for abundant RNAs and the enrichment progressively decreased to Class C and D targets. FPKM, fragments per kilobase of exon per million reads mapped.
Supplementary Figure 15 Bfr1p bound abundant, ER-translated mRNAs.
a) Plot of the fraction of ER-translated mRNAs (> 2-fold enrichment, n = 736), obtained from a published ER-specific ribosome profiling experiment42 (log2(ubc6.7mchx) enrichment), that were tagged by Bfr1p (422 mRNAs). b) Plots of the RNA abundance (FPKM) of the indicated groups of mRNAs (Tukey whiskers indicated). Of the mRNAs specifically translated at the ER, those tagged by Bfr1p were significantly more abundant than those not tagged by Bfr1p (Fisher-Pitman permutation test, P < 10−6). FPKM, fragments per kilobase of exon per million reads mapped.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 1963 kb)
Supplementary Data 1
Comprehensive GO Term enrichments for Puf3p targets. (XLSX 25 kb)
Supplementary Data 2
Comprehensive GO Term enrichments for Bfr1p targets. (XLSX 76 kb)
Supplementary Data 3
Comprehensive data file for Puf3p RNA Tagging. (XLSX 285 kb)
Supplementary Data 4
Comprehensive data file for Bfr1p RNA Tagging. (XLSX 601 kb)
Supplementary Data 5
Comprehensive data file for the control strain (BY4742). (XLSX 77 kb)
Supplementary Data 6
RNA-seq data of S. cerevisiae (BY4742) used for RNA abundance analyses and comparisons. (XLSX 265 kb)
Source data
Rights and permissions
About this article
Cite this article
Lapointe, C., Wilinski, D., Saunders, H. et al. Protein-RNA networks revealed through covalent RNA marks. Nat Methods 12, 1163–1170 (2015). https://doi.org/10.1038/nmeth.3651
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3651
This article is cited by
-
Non-coding RNAs in cancer: platforms and strategies for investigating the genomic “dark matter”
Journal of Experimental & Clinical Cancer Research (2020)
-
Methods to study RNA–protein interactions
Nature Methods (2019)
-
Identification of RNA-binding protein targets with HyperTRIBE
Nature Protocols (2018)
-
Of TRIBE and RNA tagging
Nature Methods (2016)
-
Exploring protein–RNA interactions with RNA Tagging
Nature Reviews Genetics (2016)