Abstract
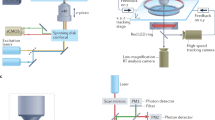
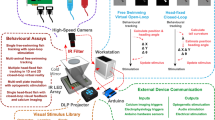
In order to localize the neural circuits involved in generating behaviors, it is necessary to assign activity onto anatomical maps of the nervous system. Using brain registration across hundreds of larval zebrafish, we have built an expandable open-source atlas containing molecular labels and definitions of anatomical regions, the Z-Brain. Using this platform and immunohistochemical detection of phosphorylated extracellular signal–regulated kinase (ERK) as a readout of neural activity, we have developed a system to create and contextualize whole-brain maps of stimulus- and behavior-dependent neural activity. This mitogen-activated protein kinase (MAP)-mapping assay is technically simple, and data analysis is completely automated. Because MAP-mapping is performed on freely swimming fish, it is applicable to studies of nearly any stimulus or behavior. Here we demonstrate our high-throughput approach using pharmacological, visual and noxious stimuli, as well as hunting and feeding. The resultant maps outline hundreds of areas associated with behaviors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fero, K., Yokogawa, T. & Burgess, H.A. The behavioral repertoire of larval zebrafish. in Neuromethods Vol. 52 (eds. Kalueff, A.V. & Cachat, J.M.) 249–291 (Humana Press, 2010).
Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M. & Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Panier, T. et al. Fast functional imaging of multiple brain regions in intact zebrafish larvae using selective plane illumination microscopy. Front. Neural Circuits 7, 65 (2013).
Fosque, B.F. et al. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760 (2015).
Nava, S.S., An, S. & Hamil, T. Visual detection of UV cues by adult zebrafish (Danio rerio). J. Vis. 11, 2 (2011).
Naumann, E.A., Kampff, A.R., Prober, D.A., Schier, A.F. & Engert, F. Monitoring neural activity with bioluminescence during natural behavior. Nat. Neurosci. 13, 513–520 (2010).
Guzowski, J.F. et al. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol. 15, 599–606 (2005).
Baraban, S.C., Taylor, M.R., Castro, P.A. & Baier, H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131, 759–768 (2005).
Ellis, L.D., Seibert, J. & Soanes, K.H. Distinct models of induced hyperactivity in zebrafish larvae. Brain Res. 1449, 46–59 (2012).
Okuyama, T. et al. Induction of c-fos transcription in the medaka brain (Oryzias latipes) in response to mating stimuli. Biochem. Biophys. Res. Commun. 404, 453–457 (2011).
Xiu, J. et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat. Neurosci. 17, 1552–1559 (2014).
Hussain, A. et al. High-affinity olfactory receptor for the death-associated odor cadaverine. Proc. Natl. Acad. Sci. USA 110, 19579–19584 (2013).
Kovács, K. Measurement of immediate-early gene activation—c-fos and beyond. J. Neuroendocrinol. 20, 665–672 (2008).
Morgan, J.I., Cohen, D.R., Hempstead, J.L. & Curran, T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science 237, 192–197 (1987).
Cancedda, L. et al. Patterned vision causes CRE-mediated gene expression in the visual cortex through PKA and ERK. J. Neurosci. 23, 7012–7020 (2003).
Ji, R.R., Baba, H., Brenner, G.J. & Woolf, C.J. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 2, 1114–1119 (1999).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Rosen, L.B., Ginty, D.D., Weber, M.J. & Greenberg, M.E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12, 1207–1221 (1994).
Thomas, G.M. & Huganir, R.L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183 (2004).
Itoh, M., Yamamoto, T., Nakajima, Y. & Hatta, K. Multistepped optogenetics connects neurons and behavior. Curr. Biol. 24, R1155–R1156 (2014).
Dai, Y. et al. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 22, 7737–7745 (2002).
Arrenberg, A.B. & Driever, W. Integrating anatomy and function for zebrafish circuit analysis. Front. Neural Circuits 7, 74 (2013).
Ronneberger, O. et al. ViBE-Z: a framework for 3D virtual colocalization analysis in zebrafish larval brains. Nat. Methods 9, 735–742 (2012).
Mueller, T. & Wullimann, M.F. Atlas of Early Zebrafish Brain Development (Elsevier, 2005).
Rohlfing, T. & Maurer, C.R. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Trans. Inf. Technol. Biomed. 7, 16–25 (2003).
Jefferis, G.S.X.E. et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128, 1187–1203 (2007).
Portugues, R., Feierstein, C.E., Engert, F. & Orger, M.B. Whole-brain activity maps reveal stereotyped, distributed networks for visuomotor behavior. Neuron 81, 1328–1343 (2014).
Orger, M.B., Kampff, A.R., Severi, K.E., Bollmann, J.H. & Engert, F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat. Neurosci. 11, 327–333 (2008).
Huang, K.-H., Ahrens, M.B., Dunn, T.W. & Engert, F. Spinal projection neurons control turning behaviors in zebrafish. Curr. Biol. 23, 1566–1573 (2013).
Pearson, G. et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 (2001).
Fernandes, A.M. et al. Deep brain photoreceptors control light-seeking behavior in zebrafish larvae. Curr. Biol. 22, 2042–2047 (2012).
Satou, C. et al. Transgenic tools to characterize neuronal properties of discrete populations of zebrafish neurons. Development 140, 3927–3931 (2013).
Tabor, K.M. et al. Direct activation of the Mauthner cell by electric field pulses drives ultra-rapid escape responses. J. Neurophysiol. 112, 834–844 (2014).
Kimmel, C.B., Patterson, J. & Kimmel, R.O. The development and behavioral characteristics of the startle response in the zebra fish. Dev. Psychobiol. 7, 47–60 (1974).
Prober, D.A. et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J. Neurosci. 28, 10102–10110 (2008).
Amaral, D.G. & Sinnamon, H.M. The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog. Neurobiol. 9, 147–196 (1977).
Lacoste, A.M.B. et al. A convergent and essential interneuron pathway for Mauthner-cell-mediated escapes. Curr. Biol. 25, 1526–1534 (2015).
Bianco, I.H., Kampff, A.R. & Engert, F. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front. Syst. Neurosci. 5, 101 (2011).
Gahtan, E., Tanger, P. & Baier, H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J. Neurosci. 25, 9294–9303 (2005).
Edwards, G.L. & Ritter, R.C. Ablation of the area postrema causes exaggerated consumption of preferred foods in the rat. Brain Res. 216, 265–276 (1981).
van de Ven, V.G., Formisano, E., Prvulovic, D., Roeder, C.H. & Linden, D.E.J. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum. Brain Mapp. 22, 165–178 (2004).
Freeman, J. et al. Mapping brain activity at scale with cluster computing. Nat. Methods 11, 941–950 (2014).
McKeown, M.J. et al. Analysis of fMRI data by blind separation into independent spatial components. Hum. Brain Mapp. 6, 160–188 (1998).
Hyvärinen, A. & Oja, E. Independent component analysis: algorithms and applications. Neural Netw. 13, 411–430 (2000).
Dreosti, E., Llopis, N.V., Carl, M., Yaksi, E. & Wilson, S.W. Left-right asymmetry is required for the habenulae to respond to both visual and olfactory stimuli. Curr. Biol. 24, 440–445 (2014).
Bianco, I.H. & Wilson, S.W. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Phil. Trans. R. Soc. Lond. B 364, 1005–1020 (2009).
Wullimann, M.F., Rupp, B. & Reichert, H. Neuroanatomy of the Zebrafish Brain. (Birkhauser, 1996).
Liu, J. et al. Evolutionarily conserved regulation of hypocretin neuron specification by Lhx9. Development 142, 1113–1124 (2015).
Wu, G.Y., Deisseroth, K. & Tsien, R.W. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat. Neurosci. 4, 151–158 (2001).
Ha, S. & Redmond, L. ERK mediates activity dependent neuronal complexity via sustained activity and CREB-mediated signaling. Dev. Neurobiol. 68, 1565–1579 (2008).
Scott, E.K. et al. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323–326 (2007).
Wen, L. et al. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev. Biol. 314, 84–92 (2008).
McLean, D.L., Fan, J., Higashijima, S.-I., Hale, M.E. & Fetcho, J.R. A topographic map of recruitment in spinal cord. Nature 446, 71–75 (2007).
Higashijima, S., Hotta, Y. & Okamoto, H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J. Neurosci. 20, 206–218 (2000).
Ben Fredj, N.B. et al. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J. Neurosci. 30, 10939–10951 (2010).
Shin, J., Park, H.-C., Topczewska, J.M., Mawdsley, D.J. & Appel, B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 25, 7–14 (2003).
Lambert, A.M., Bonkowsky, J.L. & Masino, M.A. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J. Neurosci. 32, 13488–13500 (2012).
Fujimoto, E., Stevenson, T.J., Chien, C.-B. & Bonkowsky, J.L. Developmental biology. Dev. Biol. 352, 393–404 (2011).
Coffey, C.M. et al. Novel oxytocin gene expression in the hindbrain is induced by alcohol exposure: transgenic zebrafish enable visualization of sensitive neurons. PLoS One 8, e53991 (2013).
Lillesaar, C., Stigloher, C., Tannhäuser, B., Wullimann, M.F. & Bally-Cuif, L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1expression. J. Comp. Neurol. 512, 158–182 (2009).
Parsons, M.J. et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 126, 898–912 (2009).
Scott, E.K. & Baier, H. The cellular architecture of the larval zebrafish tectum, as revealed by gal4 enhancer trap lines. Front. Neural Circuits 3, 13 (2009).
Kimmel, C.B., Powell, S.L. & Metcalfe, W.K. Brain neurons which project to the spinal cord in young larvae of the zebrafish. J. Comp. Neurol. 205, 112–127 (1982).
Bae, Y.-K. et al. Developmental biology. Dev. Biol. 330, 406–426 (2009).
Inoue, D. & Wittbrodt, J. One for all—a highly efficient and versatile method for fluorescent immunostaining in fish embryos. PLoS One 6, e19713 (2011).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
Ahrens, M.B. et al. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485, 471–477 (2012).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Zolessi, F.R., Poggi, L., Wilkinson, C.J., Chien, C.-B. & Harris, W.A. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 1, 2 (2006).
Kay, J.N., Finger-Baier, K.C., Roeser, T., Staub, W. & Baier, H. Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron 30, 725–736 (2001).
Valente, A., Huang, K.H., Portugues, R. & Engert, F. Ontogeny of classical and operant learning behaviors in zebrafish. Learn. Mem. 19, 170–177 (2012).
Ostrovsky, A., Cachero, S. & Jefferis, G. Clonal analysis of olfaction in Drosophila: image registration. Cold Spring Harb. Protoc. 2013, 347–349 (2013).
Lister, J.A., Robertson, C.P., Lepage, T., Johnson, S.L. & Raible, D.W. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757–3767 (1999).
Amunts, K., Schleicher, A. & Zilles, K. Cytoarchitecture of the cerebral cortex—more than localization. Neuroimage 37, 1061–1065 (2007).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Calhoun, V.D., Adali, T., Pearlson, G.D. & Pekar, J.J. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum. Brain Mapp. 13, 43–53 (2001).
Acknowledgements
We are grateful to M. Wullimann (Ludwig Maximilian University) for his critical and detailed input regarding the identification of regions for the Z-Brain segmentation, Y. Yoshihara (RIKEN Brain Science Institute) and T. Okuyama (University of Tokyo) for pointing us to the pERK antibody, G. Jefferis (MRC Laboratory of Molecular Biology) for help with brain registrations, M. Nikitchenko (Harvard) for help with computational and web resources, A. Douglass (University of Utah) and J. Wortzman (Harvard) for creation of the Tg(UAS:GCaMP5G) line, D. Prober (Caltech) for sharing the Tg(hcrt:mRFP) and Tg(qrfp:GFP) lines before publication, M. Hasemeyer (Harvard) for many helpful discussions, and the many members of the zebrafish community who shared their transgenic fish lines. Funding was provided by an HFSP Long-Term fellowship (LT000772/2012-L to O.R.); the Agency for Science, Technology and Research, Singapore (C.L.W.); a Marie Curie Fellowship (E.A.N.); the Swartz Foundation (J.E.F.); the Simons Foundation (SCGB award 325207 to F.E.); and NIH grants R01 HL109525, U01 MH105960 (both to A.F.S.), R24 NS086601, U01 NS090449 and DP1 NS082121-02 (to F.E.).
Author information
Authors and Affiliations
Contributions
O.R., F.E. and A.F.S. conceived of the project. O.R. performed most experiments and data analysis. C.L.W., E.A.N., D.S. and A.M.B.L. also performed experiments. E.A.N., J.E.F. and R.P. also analyzed data. D.S. and C.R. created new transgenic fish strains. O.N. built the website. O.R., F.E. and A.F.S. wrote the paper, with input from all other authors. F.E. and A.F.S. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Qualitative analyses of Z-Brain registration accuracy.
(a) Images of the post-registration positioning of Hcrt and Qrfp expressing cells in the hypothalamus from 15 different Tg(Hcrt:RFP);Tg(Qrfp:GFP)48 double transgenic fish, and the mean-signal across all fish, highlighting the tight overlap of cell-occupied territories across fish and the differential territories of these adjacent cell types. Images are maximum intensity z-projections over the 21 slices (42um) (b) Examples of the staining patterns of 14 different labels in the tectal neuropil, revealing clear and distinct banding patterns. Images are all from the same x-plane in the Z-Brain. (c) Convergence of multiple markers labeling Mauthner cell (MC) circuitry in the Z-Brain. Reticulospinal backfills and Tg(S1181t:Gal4; uas:Kaede)51 label the Mautner cell soma and axon. Tg(-6.7FRHcrtR:Gal4);Tg(uasKaede)37 and anti-Znp1 label the MC axon cap (AxC), and anti-Glycine receptor labels the surface of the MC. Images are all from the same z-plane in the Z-Brain. Scale bars, 50 μm.
Supplementary Figure 2 Examples of the successful registration of brains imaged pre-fixation and post-fixation.
Tg(Elavl3:GCaMP5G)2 fish were imaged live by 2-photon microscopy to record calcium activity. Fish were then fixed, and immunostained for pERK and tERK (not shown), and the Tg(Elavl3:GCaMP5G) transgene was re-imaged by confocal microscopy. The post-fixation image data was then registered into the anatomical stack acquired during live imaging using CMTK, and the imaging slice was re-identified using 3D cross correlation analysis. This resulted in cellular resolution overlap between the pre-and post fixation data. Shown are examples from two different fish. Scale bars, 50 μm.
Supplementary Figure 3 Discriminability and cell-type promiscuity of the pERK indicator.
(a) The Reciever Operator Characteristic plots for 4 different fish, comparing the ability of pERK to discriminate between true and false positives, where a ROI with >30 s of detected Ca2+ activity is considered to be a true positive and an active cell. Strong leftward deviation from the diagonal line indicates good discriminability in all four fish analyzed. (b) The area under the ROC curve (AUC) yields a number between 0 and 1, representing the probability that the pERK will be higher in a randomly chosen active neuron than in a randomly chosen silent neuron. By plotting the AUC as a function of the threshold of required activity observed during Ca2+ imaging to classify a cell as ‘active’, we see that the AUC increases as we increase this stringency on activity. We observe values of nearly chance (0.5) for very low activity thresholds (<1 s), while after 10 and 30 s thresholds we see values of ~0.7 and ~0.8, and we observe perfect discrimination in Fish 2 at the 41-s threshold. This indicates that the discriminability of the pERK indicator increases with increasing levels of activity. (c) Examples of Tg(Vglut2a:GFP)64 positive (arrows) and negative (arrowheads) cells exhibiting high pERK levels in the Habenula (Hab) and Telencephalon (Tel). (d) Examples of a Tg(Glyt2:GFP)53 positive (arrow) and negative (arrowhead) cell exhibiting high pERK levels in the hindbrain.
Supplementary Figure 4 Z-Brain analyses of OMR-induced activity patterns showing the activation of anterior-hindbrain GABAergic neurons.
Virtual colocalization analysis, comparing the OMR-induced activity in the medial anterior hindbrain (m-aHB) and lateral anterior hindbrain (l-aHB) to the Tg(Gad1b:GFP)32 label. The MAP-Map activity patterns are shown as outlines of the activated areas. (b) Comparison of pERK levels in Gad1B-positive cells in the m-aHB and l-aHB of Tg(Gad1B:GFP) fish, presented with gratings moving to the right. Shown are histograms, revealing significantly increased pERK levels on the right side of the brain (p=4.3x10−25, and p=8.2x10−15 for the m-aHB and l-aHB respectively, Mann-Whitney test, n=8 fish). The inset shows the results for non-GFP labeled cells, which do not show such a strong (although still significant) shift in distribution (p=6.9x10−4, and p=3.6x10−3 for the m-aHB and l-aHB, respectively). Scale bar, 50 μm.
Supplementary Figure 5 Virtual colocalization of Z-Brain labels and hunting- and feeding-induced activity.
(a) MAP-Map activity induced by exposure to paramecia (Fig. 4i) is present in the vicinity of the nucleus of the medial longitudinal fascicle (nucMLF), which partially overlaps with the MeL neurons and surrounding neuropil anterior (arrowheads) and posterior (arrows) to the cells. (b) Strong activation of the area postrema, virtually overlapping with the noradrenergic neurons labeled by both Tg(etVmat2:GFP)52 and anti-TH Z-Brain labels. (c) Suppressive signals in the dorsal-caudal hindbrain (dcHB) overlapped with two stripes of neurons labeled by Tg(Glyt2:GFP)53, which are referred to as Stripe 2 and Stripe 3 in the Z-Brain. Scale bars, 50 μm.
Supplementary Figure 6 Independent components retrieved from the analysis of 820 pERK-stained fish.
Shown are the Z and X maximum intensity projections of the z-score values of the 30 independent components, linearly mapped between z-score values of 1 and 4. Green and magenta colors represent positive and negative loadings of the independent component signals, respectively.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 (PDF 13356 kb)
Anatomical labels in the Z-Brain.
The 29 image stacks in the Z-Brain database are show, scrolling from dorsal to ventral. Stacks are in the same order as in Supplementary Table 1, moving right to left and top to bottom of the montage. (AVI 2718 kb)
Segmented anatomical regions in the Z-Brain.
Three slice views of the Z-Brain are shown in the left panel (blue box = x/y, green = x/z, red =y/z). Shown are the outlines of all the anatomical regions contained in the Z-Brain, overlayed on the Tg(HuC:H2B-RFP) mean stack label. The slice region for each box is shown by the colored tick marks. The right panel displays a rotating 3D reconstruction of the Z-Brain region outlines. Colours are assigned pseudo-randomly, biased such that the major anatomical regions are enriched for the following colours: Telencephalon \xA0 Green, Diencephalon \xA0 Cyan, Mesencephalon \xA0Yellow, Rhombencephalon \x96 Red, Spinal Cord \xA0 Magenta, Ganglia/Other (including eyes and olfactory epithelium) \xA0 Blue.\xA0 (AVI 48535 kb)
MAP-Map: exposure to pentylenetetrazol (PTZ), related to Fig. 2f.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 1941 kb)
MAP-Map: exposure to MS-222, related to Fig. 2g.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 2023 kb)
MAP-Map: exposure to MS-222, related to Fig. 2h.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 1932 kb)
MAP-Map: the optomotor response, related to Fig. 3c.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 169 kb)
MAP-Map: exposure to mustard oil, related to Fig. 4a.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 2105 kb)
MAP-Map: tap stimuli, related to Fig. 4b.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 1992 kb)
MAP-Map: exposure to noxious heat, related to Fig. 4c.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 1941 kb)
MAP-Map: electric shock stimuli, related to Fig. 4d.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 1986 kb)
Intersection of aversive MAP-Maps, related to Fig. 4e.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 460 kb)
MAP-Map: exposure to paramecia, related to Fig. 4i.
Green and magenta signals represent increased and decreased neural activity (pERK levels) over controls, respectively. Blue, green and red boxed regions show the x/y, x/z and y/z slice views. Tick marks depict the slice view position. (MP4 1938 kb)
Supplementary Data 1
Anatomical analyses of MAP-Maps using the Z-Brain. For each MAP-Map (Fig. 2f-h, 3c, 4a-e,i) there are two table outputs as separate sheets in the .xls file, reflecting green (activation) signals, and magenta (suppressive) signals, relative to controls. In each table, column 1 is the name of the ranked Z-Brain region, column 2 is the mean signal within that region, where 65535 would represent a fully saturated signals with all voxels having greater than or equal to a 0.5 delta median value, and 0 would represent no signal. In columns 3 through 12 we list the top five candidate anatomical labels that overlap with the MAP-Map signal in the region. Odd columns list the label's name, even columns indicate the enrichment for that label, which is calculated as the mean label signal within the pixels that show activity, divided by the mean label signal in the 50 pixels surrounding the region. Therefore, numbers greater than 1 indicate signal enrichment over the surrounding. (XLS 609 kb)
Supplementary Data 2
Quantification and ranking of label signals within Z-Brain regions. For each region the labels are ranked according to either the local signal enrichment calculated as the mean signal within the region, divided by the mean signal in a 50 voxel radius surrounding the region (Brightness Ratio to Surrounding sheet), or the mean signal within the region (Average Brightness sheet). (XLS 592 kb)
Rights and permissions
About this article
Cite this article
Randlett, O., Wee, C., Naumann, E. et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat Methods 12, 1039–1046 (2015). https://doi.org/10.1038/nmeth.3581
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3581
This article is cited by
-
TSA-PACT: a method for tissue clearing and immunofluorescence staining on zebrafish brain with improved sensitivity, specificity and stability
Cell & Bioscience (2023)
-
A novel small molecule, AS1, reverses the negative hedonic valence of noxious stimuli
BMC Biology (2023)
-
UBAP2 plays a role in bone homeostasis through the regulation of osteoblastogenesis and osteoclastogenesis
Nature Communications (2023)
-
An optofluidic platform for interrogating chemosensory behavior and brainwide neural representation in larval zebrafish
Nature Communications (2023)
-
Mitochondrial proteins encoded by the 22q11.2 neurodevelopmental locus regulate neural stem and progenitor cell proliferation
Molecular Psychiatry (2023)