Abstract
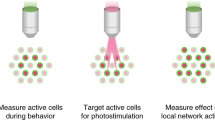
Circuit mapping requires knowledge of both structural and functional connectivity between cells. Although optical tools have been made to assess either the morphology and projections of neurons or their activity and functional connections, few probes integrate this information. We have generated a family of photoactivatable genetically encoded Ca2+ indicators that combines attributes of high-contrast photolabeling with high-sensitivity Ca2+ detection in a single-color protein sensor. We demonstrated in cultured neurons and in fruit fly and zebrafish larvae how single cells could be selected out of dense populations for visualization of morphology and high signal-to-noise measurements of activity, synaptic transmission and connectivity. Our design strategy is transferrable to other sensors based on circularly permutated GFP (cpGFP).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Chung, K. & Deisseroth, K. CLARITY for mapping the nervous system. Nat. Methods 10, 508–513 (2013).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Oh, S.W. et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014).
Patterson, G.H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Ruta, V. et al. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 468, 686–690 (2010).
Miyawaki, A. et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Tian, L. & Looger, L.L. Genetically encoded fluorescent sensors for studying healthy and diseased nervous systems. Drug Discov. Today Dis. Models 5, 27–35 (2008).
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141 (2001).
Zucker, R.S. Calcium- and activity-dependent synaptic plasticity. Curr. Opin. Neurobiol. 9, 305–313 (1999).
Zhao, Y. et al. An expanded palette of genetically encoded Ca2+ indicators. Science 333, 1888–1891 (2011).
Tian, L., Hires, S.A. & Looger, L.L. Imaging neuronal activity with genetically encoded calcium indicators. Cold Spring Harb. Protoc. 2012, 647–656 (2012).
Whitaker, M. Genetically encoded probes for measurement of intracellular calcium. Methods Cell Biol. 99, 153–182 (2010).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Tewson, P. et al. Simultaneous detection of Ca2+ and diacylglycerol signaling in living cells. PLoS ONE 7, e42791 (2012).
Sakaguchi, R. et al. A single circularly permuted GFP sensor for inositol-1,3,4,5-tetrakisphosphate based on a split PH domain. Bioorg. Med. Chem. 17, 7381–7386 (2009).
St-Pierre, F. et al. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 17, 884–889 (2014).
Marvin, J.S. et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170 (2013).
Henderson, J.N. et al. Structure and mechanism of the photoactivatable green fluorescent protein. J. Am. Chem. Soc. 131, 4176–4177 (2009).
Wu, J. et al. A long Stokes shift red fluorescent Ca2+ indicator protein for two-photon and ratiometric imaging. Nat. Commun. 5, 5262 (2014).
Yamada, Y. & Mikoshiba, K. Quantitative comparison of novel GCaMP-type genetically encoded Ca2+ indicators in mammalian neurons. Front. Cell. Neurosci. 6, 41 (2012).
Noguchi, J., Matsuzaki, M., Ellis-Davies, G.C. & Kasai, H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46, 609–622 (2005).
Araya, R., Jiang, J., Eisenthal, K.B. & Yuste, R. The spine neck filters membrane potentials. Proc. Natl. Acad. Sci. USA 103, 17961–17966 (2006).
Branco, T., Staras, K., Darcy, K.J. & Goda, Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron 59, 475–485 (2008).
Aberle, H. et al. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545–558 (2002).
Fox, L.E., Soll, D.R. & Wu, C.F. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. J. Neurosci. 26, 1486–1498 (2006).
Kohsaka, H., Okusawa, S., Itakura, Y., Fushiki, A. & Nose, A. Development of larval motor circuits in Drosophila. Dev. Growth Differ. 54, 408–419 (2012).
Kurusu, M. et al. Genetic control of development of the mushroom bodies, the associative learning centers in the Drosophila brain, by the eyeless, twin of eyeless, and dachshund genes. Proc. Natl. Acad. Sci. USA 97, 2140–2144 (2000).
Muto, A. & Kawakami, K. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun. Integr. Biol. 4, 566–568 (2011).
Shigetomi, E., Kracun, S., Sofroniew, M.V. & Khakh, B.S. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 13, 759–766 (2010).
Bulina, M.E. et al. A genetically encoded photosensitizer. Nat. Biotechnol. 24, 95–99 (2006).
Matsuda, T., Horikawa, K., Saito, K. & Nagai, T. Highlighted Ca2+ imaging with a genetically encoded 'caged' indicator. Sci. Rep. 3, 1398 (2013).
Thestrup, T. et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat. Methods 11, 175–182 (2014).
Hoi, H., Matsuda, T., Nagai, T. & Campbell, R.E. Highlightable Ca2+ indicators for live cell imaging. J. Am. Chem. Soc. 135, 46–49 (2013).
Lin, J.Y., Knutsen, P.M., Muller, A., Kleinfeld, D. & Tsien, R.Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013).
Chuong, A.S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Klapoetke, N.C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Subach, F.V. et al. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat. Methods 6, 153–159 (2009).
Subach, F.V., Patterson, G.H., Renz, M., Lippincott-Schwartz, J. & Verkhusha, V.V. Bright monomeric photoactivatable red fluorescent protein for two-color super-resolution sptPALM of live cells. J. Am. Chem. Soc. 132, 6481–6491 (2010).
Muto, A., Ohkura, M., Abe, G., Nakai, J. & Kawakami, K. Real-time visualization of neuronal activity during perception. Curr. Biol. 23, 307–311 (2013).
Ohkura, M. et al. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS ONE 7, e51286 (2012).
Sun, X.R. et al. Fast GCaMPs for improved tracking of neuronal activity. Nat. Commun. 4, 2170 (2013).
Helassa, N. et al. Ultrafast genetically encoded calcium indicators for visualizing calcium flux and action potentials. Biophys. J. 106 (suppl. 1), 242a (2014).
Beaudoin, G.M. III et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 7, 1741–1754 (2012).
Drobizhev, M., Makarov, N.S., Tillo, S.E., Hughes, T.E. & Rebane, A. Two-photon absorption properties of fluorescent proteins. Nat. Methods 8, 393–399 (2011).
Schneider, M., Barozzi, S., Testa, I., Faretta, M. & Diaspro, A. Two-photon activation and excitation properties of PA-GFP in the 720–920-nm region. Biophys. J. 89, 1346–1352 (2005).
Oron, D., Papagiakoumou, E., Anselmi, F. & Emiliani, V. Two-photon optogenetics. Prog. Brain Res. 196, 119–143 (2012).
Carroll, E.C. et al. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proc. Natl. Acad. Sci. USA 112, E776–E785 (2015).
Suster, M.L., Kikuta, H., Urasaki, A., Asakawa, K. & Kawakami, K. Transgenesis in zebrafish with the Tol2 transposon system. Methods Mol. Biol. 561, 41–63 (2009).
Zelenchuk, T.A. & Brusés, J.L. In vivo labeling of zebrafish motor neurons using an mnx1 enhancer and Gal4/UAS. Genesis 49, 546–554 (2011).
Brand, A.H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Wang, Z., Singhvi, A., Kong, P. & Scott, K. Taste representations in the Drosophila brain. Cell 117, 981–991 (2004).
Marella, S. et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295 (2006).
Hirose, M. et al. Structure of the human-heart fatty-acid-binding protein 3 in complex with the fluorescent probe 1-anilinonaphthalene-8-sulphonic acid. J. Synchrotron Radiat. 20, 923–928 (2013).
Acknowledgements
We thank C. Stanley and Z. Fu for help with molecular biology, H. Aaron for technical help with microscopy and C. Chang for fluorimeter use. We also thank R.Y. Tsien (University of California, San Diego) for the pRSETB vector, J.L. Brusés (University of Kansas) for the generous gift of the mnx1-GAL4 construct and D. Friedmann for generating the mnx1-GAL4 transgenic zebrafish line. The work was supported by US National Science Foundation (NSF) Graduate Research Fellowship (1106400; Z.L.N.), NSF Major Research Instrumentation (1041078; E.Y.I.), US National Institute of General Medical Sciences (R01 GM068552; J.C.L.) and US National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function (2PN2EY01824; E.Y.I.).
Author information
Authors and Affiliations
Contributions
S.B., E.C.C. and E.Y.I. conceived of the project. S.B. designed and constructed all the clones with help from H.O.O. S.B. performed all experiments in cultured cells and patching experiments. Z.L.N. maintained transgenic Drosophila lines and conducted larval Drosophila experiments with help from S.B. and E.C.C. B.K. conducted adult Drosophila experiments with help from S.B., E.C.C. and Z.L.N. E.C.C. conducted zebrafish experiments with help from C.M.Q. and S.B. S.S.M. purified proteins with supervision of J.C.L. and N.C.R. E.C.C. and S.B. characterized the purified proteins. S.B., E.C.C. and E.Y.I. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–20, Supplementary Tables 1 and 2 and Supplementary Note (PDF 8774 kb)
short-GCaMP3-WT
Dissociated hippocampal neuron, transfected with short-GCaMP3-WT, displaying spontaneous activity. (MOV 1703 kb)
Highlighting of HeLa cells
Sequential photoactivation of sPA-GCaMP3 transfected in HeLa cells. (MOV 3059 kb)
Highlighting of a hippocampal neuron by ssPA-GCaMP6m
Repetitive photoactivation bouts targeted at the soma of a dissociated hippocampal neuron, transfected with ssPA-GCaMP6m, yield progressive highlighting of the neuron and its processes. (MOV 8968 kb)
Highlighting a hippocampal neuron by sPA-GCaMP6f
Dissociated neuron transfected with sPA-GCaMP6f undergoing highlighting (color coded). (MOV 3851 kb)
Ca2+ activity in dendrites and spines
Ca2+ activity observed in dendrites and spines, following highlighting with sPA-GCaMP6f (MOV 6625 kb)
Simultaneous photoactivation of individual cells
Simultaneous functional highlighting of many dissociated hippocampal neurons, expressing sPA-GCaMP6m, in which spontaneous activity can be seen detected. (MOV 18498 kb)
Sequential single cell photoactivation
Sequential photoactivation of closely situated dissociated neurons, enable tracing of the neurons' processes. (MOV 15599 kb)
Connected cells
sPA-GCaMP6m highlights the cell and enables imaging Ca2+ signals from the cell's soma, dendrites and spines. (AVI 16979 kb)
Simultaneous photoactivation of individual motor neuron somata in the VNC
Color-coded Highlighting of individual somata, expressing sPA-GCaMP6f, in the ventral nerve cord of a transgenic Drosophila larvae. (AVI 45241 kb)
Zebrafish glia displaying Ca2+ activity, in vivo
In vivo imaging of Ca2+ activity in many photoactivated Müller glia in Zebrafish larvae, transiently expressing sPA-GCaMP6f (color coded). (AVI 7211 kb)
Confined Ca2+ activity in glia cells, in vivo
In vivo imaging of Ca2+ activity in a single Müller glia in Zebrafish larvae, transiently expressing sPA-GCaMP6f. The cell displays distinct patters of Ca2+ activity in different regions of the cell. (AVI 3935 kb)
Rights and permissions
About this article
Cite this article
Berlin, S., Carroll, E., Newman, Z. et al. Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging. Nat Methods 12, 852–858 (2015). https://doi.org/10.1038/nmeth.3480
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3480
This article is cited by
-
Genetically encoded photo-switchable molecular sensors for optoacoustic and super-resolution imaging
Nature Biotechnology (2022)
-
Absolute measurement of cellular activities using photochromic single-fluorophore biosensors and intermittent quantification
Nature Communications (2022)
-
Reinforcing Interdisciplinary Collaborations to Unravel the Astrocyte “Calcium Code”
Journal of Molecular Neuroscience (2022)
-
Recent advances in development of devices and probes for sensing and imaging in the brain
Science China Chemistry (2021)
-
Upconversion optogenetic micro-nanosystem optically controls the secretion of light-responsive bacteria for systemic immunity regulation
Communications Biology (2020)