Abstract
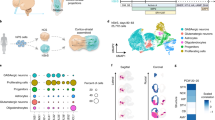
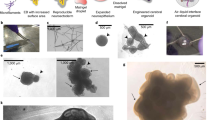
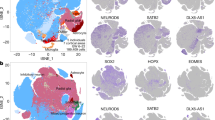
The human cerebral cortex develops through an elaborate succession of cellular events that, when disrupted, can lead to neuropsychiatric disease. The ability to reprogram somatic cells into pluripotent cells that can be differentiated in vitro provides a unique opportunity to study normal and abnormal corticogenesis. Here, we present a simple and reproducible 3D culture approach for generating a laminated cerebral cortex–like structure, named human cortical spheroids (hCSs), from pluripotent stem cells. hCSs contain neurons from both deep and superficial cortical layers and map transcriptionally to in vivo fetal development. These neurons are electrophysiologically mature, display spontaneous activity, are surrounded by nonreactive astrocytes and form functional synapses. Experiments in acute hCS slices demonstrate that cortical neurons participate in network activity and produce complex synaptic events. These 3D cultures should allow a detailed interrogation of human cortical development, function and disease, and may prove a versatile platform for generating other neuronal and glial subtypes in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Dolmetsch, R. & Geschwind, D.H. The human brain in a dish: the promise of iPSC-derived neurons. Cell 145, 831–834 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Tabar, V. & Studer, L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat. Rev. Genet. 15, 82–92 (2014).
Brennand, K.J., Simone, A., Tran, N. & Gage, F.H. Modeling psychiatric disorders at the cellular and network levels. Mol. Psychiatry 17, 1239–1253 (2012).
Pas¸ca, S.P., Panagiotakos, G. & Dolmetsch, R.E. Generating human neurons in vitro and using them to understand neuropsychiatric disease. Annu. Rev. Neurosci. 37, 479–501 (2014).
Mariani, J. et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 109, 12770–12775 (2012).
Kadoshima, T. et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 110, 20284–20289 (2013).
Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Brennand, K.J. & Gage, F.H. Modeling psychiatric disorders through reprogramming. Dis. Model. Mech. 5, 26–32 (2012).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Sarnat, H.B., Nochlin, D. & Born, D.E. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev. 20, 88–94 (1998).
Stein, J.L. et al. A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron 83, 69–86 (2014).
Kang, H.J. et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 (2011).
Miller, J.A. et al. Transcriptional landscape of the prenatal human brain. Nature 508, 199–206 (2014).
Englund, C. et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247–251 (2005).
Hansen, D.V., Lui, J.H., Parker, P.R. & Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 (2010).
Weissman, T., Noctor, S.C., Clinton, B.K., Honig, L.S. & Kriegstein, A.R. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb. Cortex 13, 550–559 (2003).
Taverna, E. & Huttner, W.B. Neural progenitor nuclei IN motion. Neuron 67, 906–914 (2010).
Meyer, G. & Goffinet, A.M. Prenatal development of reelin-immunoreactive neurons in the human neocortex. J. Comp. Neurol. 397, 29–40 (1998).
Saito, T. et al. Neocortical layer formation of human developing brains and lissencephalies: consideration of layer-specific marker expression. Cereb. Cortex 21, 588–596 (2011).
Alcamo, E.A. et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57, 364–377 (2008).
Britanova, O. et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57, 378–392 (2008).
Sugitani, Y. et al. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 16, 1760–1765 (2002).
Zeng, H. et al. Large-scale cellular-resolution gene profiling in human neocortex reveals species-specific molecular signatures. Cell 149, 483–496 (2012).
Shen, Q. et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat. Neurosci. 9, 743–751 (2006).
Bayer, S.A. & Altman, J. Neocortical Development (Raven Press, New York, 1991).
Micheva, K.D. & Smith, S.J. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55, 25–36 (2007).
Foo, L.C. et al. Development of a method for the purification and culture of rodent astrocytes. Neuron 71, 799–811 (2011).
McCarthy, K.D. & de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902 (1980).
Zamanian, J.L. et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410 (2012).
Brown, A.M. & Ransom, B.R. Astrocyte glycogen and brain energy metabolism. Glia 55, 1263–1271 (2007).
Pas¸ca, S.P. et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat. Med. 17, 1657–1662 (2011).
Brennand, K.J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Marchetto, M.C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Pfrieger, F.W. & Barres, B.A. Synaptic efficacy enhanced by glial cells in vitro. Science 277, 1684–1687 (1997).
Ullian, E.M., Sapperstein, S.K., Christopherson, K.S. & Barres, B.A. Control of synapse number by glia. Science 291, 657–661 (2001).
Krencik, R., Weick, J.P., Liu, Y., Zhang, Z.J. & Zhang, S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 29, 528–534 (2011).
Meyer, K. et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl. Acad. Sci. USA 111, 829–832 (2014).
Allen, N.J. et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414 (2012).
Eroglu, C. et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 (2009).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Dugas, J.C., Tai, Y.C., Speed, T.P., Ngai, J. & Barres, B.A. Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 26, 10967–10983 (2006).
Micheva, K.D., Busse, B., Weiler, N.C., O'Rourke, N. & Smith, S.J. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68, 639–653 (2010).
Acknowledgements
We thank R. Dolmetsch, R. O'Hara, U. Francke and J. Hallmayer for valuable scientific advice and discussions, and also acknowledge E. Engleman and the Stanford Blood Flow Cytometry Center for technical advice and support, J. Ou for assistance with RNA preparation, and D. Castaneda-Castellanos for assistance with live imaging. This work was supported by a NARSAD Young Investigator Award (Behavioral and Brain Foundation), US National Institute of Mental Health (NIMH) 1R01MH100900 and 1R01MH100900-02S1, MQ Fellow Award and Startup Funds from Stanford University (to S.P.P.); NIMH R01 MH099555-03 (to B.A.B.); NIMH T32GM007365, F30MH106261 and Bio-X Predoctoral Fellowship (to or supporting S.A.S.); NIMH 5R37 MH060233 and 5R01 MH094714 (to D.H.G.); NIH R01NS075252, R21MH099797 and R01NS092474 (to S.J.S.); and the DGIST R&D Program of the Korean Ministry of Science and ICT & Future Planning, 14-BD-16 (to C.H.K.).
Author information
Authors and Affiliations
Contributions
A.M.P., S.A.S. and S.P.P. conceived the project. A.M.P., S.A.S., L.E.C., Y.T., C.D.M., C.H.K., J.-Y.P., N.A.O'R., K.D.N., N.H., S.J.S., J.R.H., D.H.G., B.A.B. and S.P.P. planned and/or executed experiments. A.M.P., S.A.S. and S.P.P. wrote the paper with input from all authors. S.P.P. supervised all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Antibody specificity.
Panel showing the specificity of the antibodies against NEUN, GFAP, FOXG1, PAX6 (Rb), PAX6 (Mo) in negative cells (HEK293T). The last row shows background immunostaining for secondary-only conditions. All images were collected at a 500 ms exposure.
Supplementary Figure 2 Transcriptional analyses and mapping of neuronal cultures derived from hiPSCs using a monolayer approach.
The machine learning algorithm CoNTExT, which matches transcriptomes to human brain development, was used to predict the in vivo temporal identity of neural progenitors and neurons differentiated from hiPSC using a monolayer approach (adapted from Fig. 7 in Stein et. al., 2014). In contrast to the hCSs in Fig. 1e that reach up to fetal stage 6, these cultures map to earlier stages of brain development.
Stein, J.L., Torre-Ubieta, L., Tian, Y., Parikshak, N.P., Hernandez, I.A., Marchetto, M.C., Baker, D.K., Lu, D., Hinman, C.R., Lowe, J.K., Wexler, E.M., Muotri, A.R., Gage, F.H., Kosik, K.S., and Geschwind, D.H. A Quantitative Framework to Evaluate Modeling of Cortical Development by Neural Stem Cells Neuron 83, 69-86 (2014).
Supplementary Figure 3 Validation of layer specific antibodies in the human fetal cortex at PCW36.
(a) CTIP2 and SATB2. (b) TBR1. (c) CTIP2 and BRN2. (d) CTIP2, SATB2 and CUX2.
Supplementary Figure 4 Variability in the generation of hCSs.
(a) Proportion of neurons (mean ± s.e.m.) expressing CTIP2 and SATB2 at day 40 of differentiation. Multiple spheroids differentiated at the same time from one hiPSC line. Standard deviation is 2.9% for CTIP2 and 1.5% for SATB2. (b) Proportion of neurons (mean ± s.e.m.) expressing CTIP2 and SATB2 at day 76 of differentiation. The same hiPSC line was differentiated in two different experiments at two different times (multiple hCS per differentiation). Two-way ANOVA, F1,14 = 0.1940, P = 0.66 for hiPSC lines; multiple comparison test P > 0.05. (c) Proportion of neurons (mean ± s.e.m.) expressing CTIP2 and SATB2 at day 76 of differentiation. Two hiPSC lines derived from two individuals were differentiated at two different times (multiple hCS per differentiation). Two-way ANOVA, F1,14 = 1.257, P = 0.28; multiple comparison test P > 0.05.
Supplementary Figure 5 Flow cytometry analysis of hCSs.
(a) Example of scatter plots for each of the antibodies used (first three rows) and the secondary only control conditions (fourth row). The marker of interest is presented on the x-axis and the threshold gate is based on the negative control samples (cells stained with secondary antibodies alone). The y-axis represents a "dump channel", a BV-421 fluorescent channel in which the cells were not stained with any fluorophores. Any positive signals on this BV-421 channel represent highly auto-flourescent cells or false positives and were excluded from the actual positive gates. (b) Quantification of the proportion of cells expressing various markers at day 76 of in vitro differentiation as assessed by flow cytometry.
Supplementary Figure 6 Expression of activation markers in hCSs before and after exposure to serum.
hCSs plated in monolayer were cultured in Neurobasal–B27 media with our without 20% serum (FBS). After 5 days, cells were harvested and the expression of genes associated with astrocyte activation (GFAP, VIM, LCN2) was measured by qPCR (t-tests with multiple comparison corrections using the Holm-Sidak method; n = 3 for each gene, *, P < 0.05; **, P < 0.01; ***, P < 0.001).
Supplementary Figure 7 Electrophysiology (hCSs plated in monolayer).
(a) Pharmacology of synaptic currents in neurons derived in hCS and plated in monolayer (at –70 mV). The frequency of EPSCs was abolished by NBQX (25 μM) and D-AP5 (50 μM) (paired t-test, n = 11 cells, P = 0.001), and was significantly reduced by 1 μM TTX (Wilcoxon signed-rank test, n = 10, P = 0.002). (b) TTX significantly reduced the amplitude of the EPSCs (P < 0.0001, paired t-test, versus ACSF, n = 10 cells).
Supplementary Figure 8 Electrophysiology (hCS slice recordings).
(a) Representative trace of a whole-cell current-clamp recording in an acute hCS slice preparation. Current injections (6 or 12 pA steps from –65 mV) produce sustained action potential generation. (b) Representative averaged trace of 53 sEPSCs in an individual hCS neuron under control conditions. (c) EPSCs were blocked by bath application of kynurenic acid in sliced hCSs (t-test, n = 6 cells; P = 0.0008). (d) Examples of voltage clamp recordings in two different hCSs showing EPSCs after electrical stimulation in an acute hCS slice preparation. The electrical stimulation artifact is designated by a red dot. (e) EPSC frequency 1s prior compared to 2s after electrical stimulation (t-test, n = 3 cells; P = 0.02) (f) Left: Representative traces of spontaneous action potentials (top three traces) and compound EPSPs (bottom three traces). Right: Representative examples of stimulus-evoked action potentials (top three traces) and compound EPSPs (bottom three traces). The electrical stimulation artifact is designated by a red dot.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–4 (PDF 1480 kb)
Live imaging showing cell division in a radial glia inside the hCS
At day 45 in vitro, hCS were infected with a lentivirus expressing EGFP under the human GFAP promoter (LentiGFAP::EGFP). At day 52 of differentiation in vitro, hCS were sliced and VZ-like regions were imaged at 37°C with a Leica SP8 confocal microscope for up to 3 hours (maximum projection of a z-stack, one frame collected every 10 min). (AVI 1022 kb)
Calcium imaging in hCS showing spontaneous activity
hCS were loaded with the calcium indicator Fluo-4 for 30 min, sectioned in half and imaged with a Zeiss confocal L710 microscope. (AVI 554 kb)
Rights and permissions
About this article
Cite this article
Paşca, A., Sloan, S., Clarke, L. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12, 671–678 (2015). https://doi.org/10.1038/nmeth.3415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3415
This article is cited by
-
Universal, label-free, single-molecule visualization of DNA origami nanodevices across biological samples using origamiFISH
Nature Nanotechnology (2024)
-
A cell fate decision map reveals abundant direct neurogenesis bypassing intermediate progenitors in the human developing neocortex
Nature Cell Biology (2024)
-
Profiling human brain vascular cells using single-cell transcriptomics and organoids
Nature Protocols (2024)
-
Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids
Nature Biotechnology (2024)
-
A beginner’s guide on the use of brain organoids for neuroscientists: a systematic review
Stem Cell Research & Therapy (2023)