Abstract
Serial femtosecond X-ray crystallography (SFX) has revolutionized atomic-resolution structural investigation by expanding applicability to micrometer-sized protein crystals, even at room temperature, and by enabling dynamics studies. However, reliable crystal-carrying media for SFX are lacking. Here we introduce a grease-matrix carrier for protein microcrystals and obtain the structures of lysozyme, glucose isomerase, thaumatin and fatty acid–binding protein type 3 under ambient conditions at a resolution of or finer than 2 Å.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schlichting, I. & Miao, J. Curr. Opin. Struct. Biol. 22, 613–626 (2012).
Chapman, H.N. et al. Nature 470, 73–77 (2011).
Neutze, R., Wouts, R., van der Spoel, D., Weckert, E. & Hajdu, J. Nature 406, 752–757 (2000).
Barty, A. et al. Nat. Photonics 6, 35–40 (2012).
Emma, P. et al. Nat. Photonics 4, 641–647 (2010).
Ishikawa, T. et al. Nat. Photonics 6, 540–544 (2012).
Boutet, S. et al. Science 337, 362–364 (2012).
Barends, T.R.M. Nature 505, 244–247 (2014).
Johansson, L.C. et al. Nat. Methods 9, 263–265 (2012).
Redecke, L. et al. Science 339, 227–230 (2013).
Koopmann, R. et al. Nat. Methods 9, 259–262 (2012).
Kern, J. et al. Science 340, 491–495 (2013).
Weierstall, U., Spence, J.C.H. & Doak, R.B. Rev. Sci. Instrum. 83, 035108 (2012).
Park, J., Joti, Y., Ishikawa, T. & Song, C. Appl. Phys. Lett. 103, 264101 (2013).
Weierstall, U. et al. Nat. Commun. 5, 3309 (2014).
Liu, W. et al. Science 342, 1521–1524 (2013).
White, T. A. et al. J. Appl. Cryst. 45, 335–341 (2012).
Falkner, J.C. et al. Chem. Mater. 17, 2679–2686 (2005).
Masuda, T., Ohta, K., Mikami, B. & Kitabatake, N. Acta Crystallogr. F Struct. Biol. Commun. 67, 652–658 (2011).
Hirose, M. et al. J. Synchrotron Radiat. 20, 923–928 (2013).
Kameshima, T. et al. Rev. Sci. Instrum. 85, 033110 (2014).
Tono, K. et al. New J. Phys. 15, 083035 (2013).
Yumoto, H. et al. Nat. Photonics 7, 43–47 (2013).
Duisenberg, A.J.M. J. Appl. Cryst. 25, 92–96 (1992).
Leslie, A.G.W. Acta Crystallogr. D Biol. Crystallogr. 62, 48–57 (2006).
Powell, H.R. Acta Crystallogr. D Biol. Crystallogr. 55, 1690–1695 (1999).
Kabsch, W. J. Appl. Cryst. 26, 795–800 (1993).
Kabsch, W. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A.J. et al. J. Appl. Cryst. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Collaborative Computational Project, Number 4. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Adams, P.D. et al. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Acknowledgements
The XFEL experiments were carried out at the BL3 of SACLA with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal nos. 2012B8036, 2013A8039, 2013A8040, 2013B8044, 2013B8045 and 2014A8032). This work was supported by RIKEN, by the X-ray Free-Electron Laser Priority Strategy Program (MEXT) and partly by Research Acceleration Program of Japan Science and Technology Agency. The sample preparation of FABP3 was supported by the JST-ERATO Murata Lipid Active Structure Project. The authors thank the SACLA beamline staff for technical assistance, K. Diederichs for help with data analysis and A. Nisbet for careful reading of the manuscript.
Author information
Authors and Affiliations
Contributions
M. Sugahara introduced grease-matrix extrusion scheme. M. Sugahara, E.M., E.N., M. Suzuki, T.T. and T.M. performed data collection, data processing, structure refinements (lysozyme: M. Sugahara and E.N.; glucose isomerase: E.N. and T.T.; thaumatin: T.M.; FABP3: E.M. and M. Suzuki). E.M., E.N., T.T., T.M., T.S., Y.T., C. Suno, K.I., D.P., K.K., S.S., M.M. and T.I. developed the microcrystal sample preparations and prepared samples (lysozyme: T.S., Y.T., C. Suno, K.I., D.P., E.N. and T.T.; glucose isomerase: E.N. and T.T.; thaumatin: T.M.; FABP3: E.M., K.K., S.S., M.M. and T.I.). E.N. and R.T. designed and developed the injection method. K.T. and M.Y. developed the DAPHNIS. K.T., C. Song, J.P., T.K., T.H., Y.J. and M.Y. developed the SFX systems including injectors. M. Sugahara, E.N. and C. Song wrote the manuscript with input from all the coauthors. S.I. coordinated the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
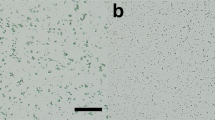
Supplementary Figure 1 Protein microcrystals used for SFX measurements.
(a) lysozyme, (b) glucose isomerase, (c) thaumatin and (d) FABP3 crystals. Scale bars represent 20 μm.
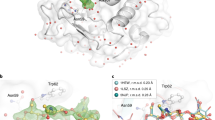
Supplementary Figure 2 Room-temperature structure of glucose isomerase.
(a) A typical diffraction pattern from an individual microcrystal. Resolution at the edges corresponds to ~1.6 Å. (b) A close-up view of glucose isomerase structure with (2Fo – Fc) electron-density map (contoured at 1.0σ). This figure was drawn with the program PyMol (http://www.pymol.org).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 and Supplementary Tables 1 and 2
Grease matrix carrier of proteins and micro-extrusion.
The crystal solution was dispensed into mineral oil–based grease, and then mixed. The crystal-containing grease was inserted into a dispenser tip. After the tip was centrifuged, the sample was loaded into a syringe. The grease produced a stable flow during the SFX experiment. (AVI 10763 kb)
Rights and permissions
About this article
Cite this article
Sugahara, M., Mizohata, E., Nango, E. et al. Grease matrix as a versatile carrier of proteins for serial crystallography. Nat Methods 12, 61–63 (2015). https://doi.org/10.1038/nmeth.3172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3172
This article is cited by
-
Oxygen-evolving photosystem II structures during S1–S2–S3 transitions
Nature (2024)
-
Growing and making nano- and microcrystals
Nature Protocols (2023)
-
Mapping protein dynamics at high spatial resolution with temperature-jump X-ray crystallography
Nature Chemistry (2023)
-
Beef tallow injection matrix for serial crystallography
Scientific Reports (2022)
-
Excited-state intermediates in a designer protein encoding a phototrigger caught by an X-ray free-electron laser
Nature Chemistry (2022)