Abstract
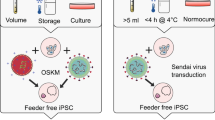
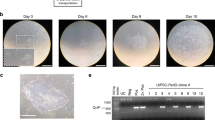
Human neural stem cells hold great promise for research and therapy in neural disease. We describe the generation of integration-free and expandable human neural progenitor cells (NPCs). We combined an episomal system to deliver reprogramming factors with a chemically defined culture medium to reprogram epithelial-like cells from human urine into NPCs (hUiNPCs). These transgene-free hUiNPCs can self-renew and can differentiate into multiple functional neuronal subtypes and glial cells in vitro. Although functional in vivo analysis is still needed, we report that the cells survive and differentiate upon transplant into newborn rat brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Aboody, K., Capela, A., Niazi, N., Stern, J.H. & Temple, S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron 70, 597–613 (2011).
Breunig, J.J., Haydar, T.F. & Rakic, P. Neural stem cells: historical perspective and future prospects. Neuron 70, 614–625 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Hu, B.Y. et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 107, 4335–4340 (2010).
Park, I.H. et al. Disease-specific induced pluripotent stem cells. Cell 134, 877–886 (2008).
Soldner, F. et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell 146, 318–331 (2011).
Israel, M.A. et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature 482, 216–220 (2012).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Yoo, A.S. et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476, 228–231 (2011).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Ambasudhan, R. et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9, 113–118 (2011).
Son, E.Y. et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218 (2011).
Qiang, L. et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell 146, 359–371 (2011).
Huang, P. et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475, 386–389 (2011).
Sekiya, S. & Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475, 390–393 (2011).
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J. & Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 (2008).
Zhou, T. et al. Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. 22, 1221–1228 (2011).
Yu, J. et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science 324, 797–801 (2009).
Liao, B. et al. MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J. Biol. Chem. 286, 17359–17364 (2011).
Ludwig, T.E. et al. Feeder-independent culture of human embryonic stem cells. Nat. Methods 3, 637–646 (2006).
Ying, Q.L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Shi, Y. et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell 2, 525–528 (2008).
Lluis, F. et al. Periodic activation of Wnt/β-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell 3, 493–507 (2008).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
Burdon, T., Stracey, C., Chambers, I., Nichols, J. & Smith, A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 210, 30–43 (1999).
Yu, J., Chau, K.F., Vodyanik, M.A., Jiang, J. & Jiang, Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS ONE 6, e17557 (2011).
Hao, J. et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem. Biol. 5, 245–253 (2010).
Kim, J. et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc. Natl. Acad. Sci. USA 108, 7838–7843 (2011).
Reynolds, B.A. & Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992).
Zhang, X. et al. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7, 90–100 (2010).
Liu, H. & Zhang, S.C. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell. Mol. Life Sci. 68, 3995–4008 (2011).
Hu, B.Y., Du, Z.W., Li, X.J., Ayala, M. & Zhang, S.C. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development 136, 1443–1452 (2009).
Thier, M. et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10, 473–479 (2012).
Ring, K.L. et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11, 100–109 (2012).
Han, D.W. et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10, 465–472 (2012).
Sheng, C. et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 22, 208–218 (2012).
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012).
Pan, G. & Pei, D. Order from chaos: single cell reprogramming in two phases. Cell Stem Cell 11, 445–447 (2012).
Esteban, M.A. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 (2010).
Esteban, M.A. et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J. Biol. Chem. 284, 17634–17640 (2009).
Acknowledgements
We thank M. Esterban for helpful suggestions, Z. Li for providing support in the initial phase of this work and members of our labs for their kind help. This work is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant nos. XDA01020202 and XDA01020401); National Basic Research Program of China, 973 Program of China (2012CB966503 and 2012CB966802); National S&T Major Special Project on Major New Drug Innovation (2011ZX09102-010); and National Natural Science Foundation of China (31200970 and 91213304). D.P. and G.P. are supported by the 100 Talents Project of Chinese Academy of Sciences, China.
Author information
Authors and Affiliations
Contributions
G.P., Lihui, W. and D.P. conceived hypotheses and designed the experiments. Lihui, W. and W.H. performed the experiments and generated data in all figures. In addition, Linli, W., D.Q., Y.X. and Z.S. performed experiments for Figure 1 and Supplementary Figure 2; H.S., W.W. and K.F.S. participated in experiments and analysis for Figure 5; X.B. provided reagents and experimental assistance for miR302–367; B.L. performed the experiments for Figure 1; and H.W. and J.H. performed the experiments for Figure 4. G.P. and D.P. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1–4 (PDF 8827 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Wang, L., Huang, W. et al. Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods 10, 84–89 (2013). https://doi.org/10.1038/nmeth.2283
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2283
This article is cited by
-
Generation of mitochondria-rich kidney organoids from expandable intermediate mesoderm progenitors reprogrammed from human urine cells under defined medium
Cell & Bioscience (2022)
-
Presenilin-1 F105C mutation leads to tau accumulation in human neurons via the Akt/mTORC1 signaling pathway
Cell & Bioscience (2022)
-
MYOCD is Required for Cardiomyocyte-like Cells Induction from Human Urine Cells and Fibroblasts Through Remodeling Chromatin
Stem Cell Reviews and Reports (2022)
-
Urine-derived induced pluripotent/neural stem cells for modeling neurological diseases
Cell & Bioscience (2021)
-
Direct Reprogramming of Somatic Cells to Neurons: Pros and Cons of Chemical Approach
Neurochemical Research (2021)