Abstract
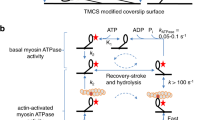
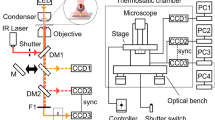
We describe a dual-trap force-clamp configuration that applies constant loads between a binding protein and an intermittently interacting biological polymer. The method has a measurement delay of only ∼10 μs, allows detection of interactions as brief as ∼100 μs and probes sub-nanometer conformational changes with a time resolution of tens of microseconds. We tested our method on molecular motors and DNA-binding proteins. We could apply constant loads to a single motor domain of myosin before its working stroke was initiated (0.2–1 ms), thus directly measuring its load dependence. We found that, depending on the applied load, myosin weakly interacted (<1 ms) with actin without production of movement, fully developed its working stroke or prematurely detached (<5 ms), thus reducing the working stroke size with load. Our technique extends single-molecule force-clamp spectroscopy and opens new avenues for investigating the effects of forces on biological processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cecconi, C., Shank, E.A., Bustamante, C. & Marqusee, S. Direct observation of the three-state folding of a single protein molecule. Science 309, 2057–2060 (2005).
Marshall, B.T. et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003).
Rief, M. et al. Myosin-V stepping kinetics: a molecular model for processivity. Proc. Natl. Acad. Sci. USA 97, 9482–9486 (2000).
Reconditi, M. et al. The myosin motor in muscle generates a smaller and slower working stroke at higher load. Nature 428, 578–581 (2004).
Laakso, J.M., Lewis, J.H., Shuman, H. & Ostap, E.M. Myosin I can act as a molecular force sensor. Science 321, 133–136 (2008).
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton. (Sinauer Associates, Inc., 2001).
Neuman, K.C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491–505 (2008).
Hinterdorfer, P. & Dufrene, Y.F. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3, 347–355 (2006).
Guo, B. & Guilford, W.H. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc. Natl. Acad. Sci. USA 103, 9844–9849 (2006).
Wiita, A.P. et al. Probing the chemistry of thioredoxin catalysis with force. Nature 450, 124–127 (2007).
Lang, M.J., Asbury, C.L., Shaevitz, J.W. & Block, S.M. An automated two-dimensional optical force clamp for single molecule studies. Biophys. J. 83, 491–501 (2002).
Greenleaf, W.J., Woodside, M.T., Abbondanzieri, E.A. & Block, S.M. Passive all-optical force clamp for high-resolution laser trapping. Phys. Rev. Lett. 95, 208102 (2005).
Litvinov, R.I., Shuman, H., Bennett, J.S. & Weisel, J.W. Binding strength and activation state of single fibrinogen-integrin pairs on living cells. Proc. Natl. Acad. Sci. USA 99, 7426–7431 (2002).
Visscher, K., Schnitzer, M.J. & Block, S.M. Single kinesin molecules studied with a molecular force clamp. Nature 400, 184–189 (1999).
Abbondanzieri, E.A., Greenleaf, W.J., Shaevitz, J.W., Landick, R. & Block, S.M. Direct observation of base-pair stepping by RNA polymerase. Nature 438, 460–465 (2005).
Finer, J.T., Simmons, R.M. & Spudich, J.A. Single myosin molecule mechanics: piconewton forces and nanometer steps. Nature 368, 113–119 (1994).
Smith, D.A., Steffen, W., Simmons, R.M. & Sleep, J. Hidden-Markov methods for the analysis of single-molecule actomyosin displacement data: the variance-Hidden-Markov method. Biophys. J. 81, 2795–2816 (2001).
Veigel, C., Molloy, J.E., Schmitz, S. & Kendrick-Jones, J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat. Cell Biol. 5, 980–986 (2003).
Capitanio, M. et al. Two independent mechanical events in the interaction cycle of skeletal muscle myosin with actin. Proc. Natl. Acad. Sci. USA 103, 87–92 (2006).
Takagi, Y., Homsher, E.E., Goldman, Y.E. & Shuman, H. Force generation in single conventional actomyosin complexes under high dynamic load. Biophys. J. 90, 1295–1307 (2006).
Kaya, M. & Higuchi, H. Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments. Science 329, 686–689 (2010).
Capitanio, M., Cicchi, R. & Pavone, F.S. Position control and optical manipulation for nanotechnology applications. Eur. Phys. J. B 46, 1–8 (2005).
Capitanio, M., Maggi, D., Vanzi, F. & Pavone, F. Fiona in the trap: the advantages of combining optical tweezers and fluorescence. J. Opt. A 9, S157 (2007).
Molloy, J.E., Burns, J.E., Kendrick-Jones, J., Tregear, R.T. & White, D.C.S. Movement and force produced by a single myosin head. Nature 378, 209–212 (1995).
Lewalle, A., Steffen, W., Stevenson, O., Ouyang, Z. & Sleep, J. Single-molecule measurement of the stiffness of the rigor myosin head. Biophys. J. 94, 2160–2169 (2008).
Nyitrai, M. & Geeves, M.A. Adenosine diphosphate and strain sensitivity in myosin motors. Phil. Trans. R. Soc. Lond. B 359, 1867–1877 (2004).
Dantzig, J.A., Hibberd, M.G., Trentham, D.R. & Goldman, Y.E. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J. Physiol. (Lond.) 432, 639–680 (1991).
Brenner, B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc. Natl. Acad. Sci. USA 88, 10490–10494 (1991).
Geeves, M.A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem. J. 274, 1–14 (1991).
Iwaki, M., Iwane, A.H., Shimokawa, T., Cooke, R. & Yanagida, T. Brownian search-and-catch mechanism for myosin-VI steps. Nat. Chem. Biol. 5, 403–405 (2009).
Piazzesi, G., Lucii, L. & Lombardi, V. The size and the speed of the working stroke of muscle myosin and its dependence on the force. J. Physiol. (Lond.) 545, 145–151 (2002).
Berg, O.G., Winter, R.B. & von Hippel, P.H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry 20, 6929–6948 (1981).
deCastro, M.J., Fondecave, R.M., Clarke, L.A., Schmidt, C.F. & Stewart, R.J. Working strokes by single molecules of the kinesin-related microtubule motor ncd. Nat. Cell Biol. 2, 724–729 (2000).
Pease, P.J. et al. Sequence-directed DNA translocation by purified FtsK. Science 307, 586–590 (2005).
Crozat, E. et al. Separating speed and ability to displace roadblocks during DNA translocation by FtsK. EMBO J. 29, 1423–1433 (2010).
Slutsky, M. & Mirny, L.A. Kinetics of protein-DNA interaction: facilitated target location in sequence-dependent potential. Biophys. J. 87, 4021–4035 (2004).
Friedman, L.J. & Gelles, J. Mechanism of transcription initiation at an activator-dependent promoter defined by single-molecule observation. Cell 148, 679–689 (2012).
Skinner, G.M., Baumann, C.G., Quinn, D.M., Molloy, J.E. & Hoggett, J.G. Promoter binding, initiation, and elongation by bacteriophage T7 RNA polymerase. A single-molecule view of the transcription cycle. J. Biol. Chem. 279, 3239–3244 (2004).
Tang, G.Q., Roy, R., Bandwar, R.P., Ha, T. & Patel, S.S. Real-time observation of the transition from transcription initiation to elongation of the RNA polymerase. Proc. Natl. Acad. Sci. USA 106, 22175–22180 (2009).
Canepari, M. et al. Functional diversity between orthologous myosins with minimal sequence diversity. J. Muscle Res. Cell Motil. 21, 375–382 (2000).
Zhan, H., Swint-Kruse, L. & Matthews, K.S. Extrinsic interactions dominate helical propensity in coupled binding and folding of the lactose repressor protein hinge helix. Biochemistry 45, 5896–5906 (2006).
Smith, S.B., Cui, Y. & Bustamante, C. Overstretching B-DNA: the elastic response of individual double-stranded and single-stranded DNA molecules. Science 271, 795–799 (1996).
Capitanio, M., Normanno, D. & Pavone, F.S. High-precision measurements of light-induced torque on absorbing microspheres. Opt. Lett. 29, 2231–2233 (2004).
Capitanio, M. et al. Calibration of optical tweezers with differential interference contrast signals. Rev. Sci. Instrum. 73, 1687–1696 (2002).
Capitanio, M., Cicchi, R. & Pavone, F.S. Continuous and time-shared multiple optical tweezers for the study of single motor proteins. Opt. Lasers Eng. 45, 450–457 (2007).
Colquhoun, D. & Sigworth, F.J. Single-Channel Recording (Plenum Press, New York, 1983).
Vanzi, F., Sacconi, L. & Pavone, F.S. Analysis of kinetics in noisy systems: application to single molecule tethered particle motion. Biophys. J. 93, 21–36 (2007).
Acknowledgements
We thank G. Belcastro for his help with Lac repressor experiments, M. Giuntini for quadrant detector photodiode electronics, and V. Lombardi and L. Gardini for discussion. This research was funded by the EU Seventh Framework Programme (FP7/2007-2013; grant agreements B0 211383, B0 228334 and B0 241526), by the Italian Ministry of University and Research (PRIN 2006 2006051323_003, FIRB 2011 RBAP11X42L006 and Flagship Project NANOMAX) and by Ente Cassa di Risparmio di Firenze to F.S.P. and by the EU Seventh Framework Programme (FP7/2007-2013; grant agreement 223576, Combating age-related muscle weakness (MYOAGE)) to R.B.
Author information
Authors and Affiliations
Contributions
M. Capitanio conceived and designed the ultrafast force clamp, performed experiments, analyzed data and wrote the paper. M.M. prepared samples and contributed to myosin experiments. D.B. contributed to setting up the ultrafast force clamp and performing myosin experiments. C.M. prepared samples and performed LacI experiments. F.V. supervised LacI experiments and wrote the paper. R.B. and M. Canepari supervised myosin experiments. R.B. contributed to writing the paper. F.S.P. supervised the design of the ultrafast force-clamp experiments and the whole project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Notes 1–8, Supplementary Results (PDF 1616 kb)
Rights and permissions
About this article
Cite this article
Capitanio, M., Canepari, M., Maffei, M. et al. Ultrafast force-clamp spectroscopy of single molecules reveals load dependence of myosin working stroke. Nat Methods 9, 1013–1019 (2012). https://doi.org/10.1038/nmeth.2152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2152
This article is cited by
-
A mutation in switch I alters the load-dependent kinetics of myosin Va
Nature Communications (2023)
-
Calculating the force-dependent unbinding rate of biological macromolecular bonds from force-ramp optical trapping assays
Scientific Reports (2022)
-
α-catenin switches between a slip and an asymmetric catch bond with F-actin to cooperatively regulate cell junction fluidity
Nature Communications (2022)
-
Single molecule turnover of fluorescent ATP by myosin and actomyosin unveil elusive enzymatic mechanisms
Communications Biology (2021)
-
Ultrafast viscosity measurement with ballistic optical tweezers
Nature Photonics (2021)