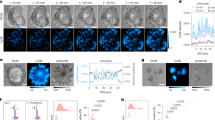
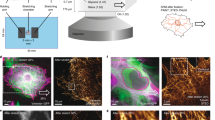
Abstract
We report a fluorescence-based turn-on sensor for mapping the mechanical strain exerted by specific cell-surface proteins in living cells. The sensor generates force maps with high spatial and temporal resolution using conventional fluorescence microscopy. We demonstrate the approach by mapping mechanical forces during the early stages of regulatory endocytosis of the ligand-activated epidermal growth factor receptor (EGFR).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vogel, V. & Sheetz, M. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006).
DuFort, C.C., Paszek, M.J. & Weaver, V.M. Nat. Rev. Mol. Cell Biol. 12, 308–319 (2011).
Dufrene, Y.F. et al. Nat. Methods 8, 123–127 (2011).
Muller, D.J., Helenius, J., Alsteens, D. & Dufrene, Y.F. Nat. Chem. Biol. 5, 383–390 (2009).
Grashoff, C. et al. Nature 466, 263–266 (2010).
Iwai, S. & Uyeda, T.Q.P. Proc. Natl. Acad. Sci. USA 105, 16882–16887 (2008).
Meng, F. & Sachs, F. J. Cell Sci. 124, 261–269 (2011).
Oesterhelt, F., Rief, M. & Gaub, H.E. New J. Phys. 1, 6.1–6.11 (1999).
Kienberger, F. et al. Single Molecules 1, 123–128 (2000).
Roberts, M.J., Bentley, M.D. & Harris, J.M. Adv. Drug Deliv. Rev. 54, 459–476 (2002).
Harder, P., Grunze, M., Dahint, R., Whitesides, G.M. & Laibinis, P.E. J. Phys. Chem. B 102, 426–436 (1998).
Goh, L.K., Huang, F., Kim, W., Gygi, S. & Sorkin, A. J. Cell Biol. 189, 871–883 (2010).
Martin, A.C., Welch, M.D. & Drubin, D.G. Nat. Cell Biol. 8, 826–833 (2006).
Salaita, K. et al. Science 327, 1380–1385 (2010).
de Gennes, P.G. Macromolecules 13, 1069–1075 (1980).
Bouchiat, C. et al. Biophys. J. 76, 409–413 (1999).
Sulchek, T.A. et al. Proc. Natl. Acad. Sci. USA 102, 16638–16643 (2005).
Saffarian, S., Cocucci, E. & Kirchhausen, T. PLoS Biol. 7, e1000191 (2009).
Clack, N.G., Salaita, K. & Groves, J.T. Nat. Biotechnol. 26, 825–830 (2008).
Nair, P.M., Salaita, K., Petit, R.S. & Groves, J.T. Nat. Protoc. 6, 523–539 (2011).
Galush, W.J., Nye, J.A. & Groves, J.T. Biophys. J. 95, 2512–2519 (2008).
Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd edn. (Springer, New York, 2006).
Sage, D., Neumann, F.R., Hediger, F., Gasser, S.M. & Unser, M. IEEE Trans. Image Process. 14, 1372–1383 (2005).
Acknowledgements
We thank A. Mattheyses (Emory University) for the CLC-eGFP plasmid and R. Nahta (Emory University Winship Cancer Institute) for the HCC1143 cells. We acknowledge the Emory University Winship Cancer Institute for support. K.S.S. acknowledges the Georgia Cancer Coalition Cancer Research Award for its support.
Author information
Authors and Affiliations
Contributions
D.R.S. adapted the FRET surface sensor for use with human cells expressing the EGFR and performed the majority of the human cell experiments. C.J. developed the force sensor and performed the quantitative characterization of the zero-force sensor conformation and its components. S.S.M. optimized and performed the CLC-eGFP transfections. K.S.S. devised the overall experimental strategy. D.R.S., C.J. and K.S.S. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 10105 kb)
Supplementary Video 1
Animation showing the mechanism of sensor function. (AVI 3308 kb)
Supplementary Video 2
Movie showing cell activation of the force sensor. (AVI 513 kb)
Supplementary Video 3
Movie showing clathrin colocalization with force sensor activation. (AVI 4616 kb)
Rights and permissions
About this article
Cite this article
Stabley, D., Jurchenko, C., Marshall, S. et al. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat Methods 9, 64–67 (2012). https://doi.org/10.1038/nmeth.1747
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1747
This article is cited by
-
Polarized focal adhesion kinase activity within a focal adhesion during cell migration
Nature Chemical Biology (2023)
-
Molecular mechanocytometry using tension-activated cell tagging
Nature Methods (2023)
-
Hydrogel-based molecular tension fluorescence microscopy for investigating receptor-mediated rigidity sensing
Nature Methods (2023)
-
Intracellular tension sensor reveals mechanical anisotropy of the actin cytoskeleton
Nature Communications (2023)
-
Single-molecule characterization of subtype-specific β1 integrin mechanics
Nature Communications (2022)