Abstract
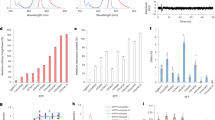
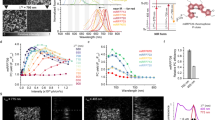
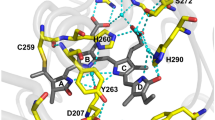
We report a photoswitchable monomeric Orange (PSmOrange) protein that is initially orange (excitation, 548 nm; emission, 565 nm) but becomes far-red (excitation, 636 nm; emission, 662 nm) after irradiation with blue-green light. Compared to its parental orange proteins, PSmOrange has greater brightness, faster maturation, higher photoconversion contrast and better photostability. The red-shifted spectra of both forms of PSmOrange enable its simultaneous use with cyan-to-green photoswitchable proteins to study four intracellular populations. Photoconverted PSmOrange has, to our knowledge, the most far-red excitation peak of all GFP-like fluorescent proteins, provides diffraction-limited and super-resolution imaging in the far-red light range, is optimally excited with common red lasers, and can be photoconverted subcutaneously in a mouse. PSmOrange photoswitching occurs via a two-step photo-oxidation process, which causes cleavage of the polypeptide backbone. The far-red fluorescence of photoconverted PSmOrange results from a new chromophore containing N-acylimine with a co-planar carbon-oxygen double bond.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lukyanov, K.A., Chudakov, D.M., Lukyanov, S. & Verkhusha, V.V. Innovation: photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 6, 885–891 (2005).
Patterson, G.H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Subach, F.V. et al. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat. Methods 6, 153–159 (2009).
Subach, F.V. et al. Bright monomeric photoactivatable red fluorescent protein for two-color super-resolution sptPALM of live cells. J. Am. Chem. Soc. 132, 6481–6491 (2010).
Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat. Protoc. 2, 2024–2032 (2007).
McKinney, S.A. et al. A bright and photostable photoconvertible fluorescent protein. Nat. Methods 6, 131–133 (2009).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Habuchi, S., Tsutsui, H., Kochaniak, A.B., Miyawaki, A. & van Oijen, A.M. mKikGR, a monomeric photoswitchable fluorescent protein. PLoS ONE 3, e3944 (2008).
Hoi, H. et al. A monomeric photoconvertible fluorescent protein for imaging of dynamic protein localization. J. Mol. Biol. 401, 776–791 (2010).
Toomre, D. & Bewersdorf, J. A new wave of cellular imaging. Annu. Rev. Cell Dev. Biol. 26, 285–314 (2010).
Stiel, A.C. et al. Generation of monomeric reversibly switchable red fluorescent proteins for far-field fluorescence nanoscopy. Biophys. J. 95, 2989–2997 (2008).
Folling, J. et al. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods 5, 943–945 (2008).
Lim, Y.T. et al. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging 2, 50–64 (2003).
Deliolanis, N.C. et al. Performance of the red-shifted fluorescent proteins in deep tissue molecular imaging applications. J. Biomed. Opt. 13, 044008 (2008).
Morozova, K.S. et al. Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscory. Biophys. J. 99, L13–L15 (2010).
Strack, R.L. et al. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry 48, 8279–8281 (2009).
Shcherbo, D. et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 418, 567–574 (2009).
Lin, M.Z. et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 16, 1169–1179 (2009).
Shcherbo, D. et al. Near-infrared fluorescent proteins. Nat. Methods 7, 827–829 (2010).
Kremers, G.J., Hazelwood, K.L., Murphy, C.S., Davidson, M.W. & Piston, D.W. Photoconversion in orange and red fluorescent proteins. Nat. Methods 6, 355–358 (2009).
Bogdanov, A.M. et al. Green fluorescent proteins are light-induced electron donors. Nat. Chem. Biol. 5, 459–461 (2009).
Shaner, N.C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 5, 545–551 (2008).
Strack, R.L. et al. A non-cytotoxic DsRed variant for whole-cell labeling. Nat. Methods 5, 955–957 (2008).
Etienne-Manneville, S. From signaling pathways to microtubule dynamics: the key players. Curr. Opin. Cell Biol. 22, 104–111 (2010).
Kuo, C., Coquoz, O., Troy, T.L., Xu, H. & Rice, B.W. Three-dimensional reconstruction of in vivo bioluminescent sources based on multispectral imaging. J. Biomed. Opt. 12, 024007 (2007).
Smith, A.M., Mancini, M.C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Shu, X., Shaner, N.C., Yarbrough, C.A., Tsien, R.Y. & Remington, S.J. Novel chromophores and buried charges control color in mFruits. Biochemistry 45, 9639–9647 (2006).
Ormö, M. et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273, 1392–1395 (1996).
Yampolsky, I.V. et al. Synthesis and properties of the chromophore of the asFP595 chromoprotein from Anemonia sulcata. Biochemistry 44, 5788–5793 (2005).
Tojo, G. & Fernández, M. Oxidation of Alcohols to Aldehydes and Ketones (Springer, New York, 2006).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K. & Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 (1989).
Chudakov, D.M. et al. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol. 22, 1435–1439 (2004).
Verkhusha, V.V. & Sorkin, A. Conversion of the monomeric red fluorescent protein into a photoactivatable probe. Chem. Biol. 12, 279–285 (2005).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Manley, S. et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 (2008).
Shroff, H. et al. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl. Acad. Sci. USA 104, 20308–20313 (2007).
Acknowledgements
We thank J. Zhang and L. Tesfa for assistance with flow cytometry, H. Xiao for help with mass-spectrometry analysis, K. Kim and G. Filonov for assistance with mouse experiments and useful discussions, M. Davidson (Florida State University) for vimentin, keratin, myosin and paxillin plasmids, and B. Glick (University of Chicago), D. Chudakov and K. Lukyanov (both from Institute of Bioorganic Chemistry) for plasmids encoding far-red fluorescent proteins. This work was supported by US National Institutes of Health (GM073913 to V.V.V. and CA100324 to J.C.) and by the National Institutes of Health Intramural Research Program including the National Institute of Biomedical Imaging and Bioengineering (to G.H.P.).
Author information
Authors and Affiliations
Contributions
O.M.S. developed the protein and characterized it in vitro. O.M.S. and G.H.P. characterized the protein in mammalian cells. O.M.S. and L.-M.T. characterized the protein in mouse models. O.M.S., Y.W. and J.S.C. performed tumor experiments. V.V.V. designed the project and, together with O.M.S., planned and discussed the project, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1–2 (PDF 3947 kb)
Rights and permissions
About this article
Cite this article
Subach, O., Patterson, G., Ting, LM. et al. A photoswitchable orange-to-far-red fluorescent protein, PSmOrange. Nat Methods 8, 771–777 (2011). https://doi.org/10.1038/nmeth.1664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1664
This article is cited by
-
Blue-shift photoconversion of near-infrared fluorescent proteins for labeling and tracking in living cells and organisms
Nature Communications (2023)
-
Chromophore reduction plus reversible photobleaching: how the mKate2 “photoconversion” works
Photochemical & Photobiological Sciences (2021)
-
Imaging of fluorescence anisotropy during photoswitching provides a simple readout for protein self-association
Nature Communications (2020)
-
mEosEM withstands osmium staining and Epon embedding for super-resolution CLEM
Nature Methods (2020)
-
Choosing proper fluorescent dyes, proteins, and imaging techniques to study mitochondrial dynamics in mammalian cells
Biophysics Reports (2017)