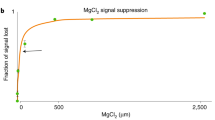
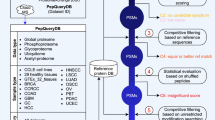
Abstract
Selected reaction monitoring (SRM) is a targeted mass spectrometric method that is increasingly used in proteomics for the detection and quantification of sets of preselected proteins at high sensitivity, reproducibility and accuracy. Currently, data from SRM measurements are mostly evaluated subjectively by manual inspection on the basis of ad hoc criteria, precluding the consistent analysis of different data sets and an objective assessment of their error rates. Here we present mProphet, a fully automated system that computes accurate error rates for the identification of targeted peptides in SRM data sets and maximizes specificity and sensitivity by combining relevant features in the data into a statistical model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
06 April 2011
In the version of this article initially published online, a 'greater than' sign was inadvertently reversed, and an author contribution was incorrectly attributed. The error has been corrected for the print, PDF and HTML versions of this article.
References
Lange, V., Picotti, P., Domon, B. & Aebersold, R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol. 4, 222 (2008).
Picotti, P., Bodenmiller, B., Mueller, L.N., Domon, B. & Aebersold, R. Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell 138, 795–806 (2009).
Wolf-Yadlin, A., Hautaniemi, S., Lauffenburger, D.A. & White, F.M. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc. Natl. Acad. Sci. USA 104, 5860–5865 (2007).
Anderson, L. & Hunter, C.L. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 5, 573–588 (2006).
Jovanovic, M. et al. A quantitative targeted proteomics approach to validate predicted microRNA targets in C. elegans. Nat. Methods 7, 837–842 (2010).
Oberg, A.L. & Vitek, O. Statistical design of quantitative mass spectrometry-based proteomic experiments. J. Proteome Res. 8, 2144–2156 (2009).
Addona, T.A. et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat. Biotechnol. 27, 633–641 (2009).
Whiteaker, J.R. et al. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J. Proteome Res. 6, 3962–3975 (2007).
Keshishian, H., Addona, T., Burgess, M., Kuhn, E. & Carr, S.A. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol. Cell. Proteomics 6, 2212–2229 (2007).
Keshishian, H. et al. Quantification of cardiovascular biomarkers in patient plasma by targeted mass spectrometry and stable isotope dilution. Mol. Cell. Proteomics 8, 2339–2349 (2009).
Mallick, P. et al. Computational prediction of proteotypic peptides for quantitative proteomics. Nat. Biotechnol. 25, 125–131 (2007).
Deutsch, E.W., Lam, H. & Aebersold, R. PeptideAtlas: a resource for target selection for emerging targeted proteomics workflows. EMBO Rep. 9, 429–434 (2008).
Lange, V. et al. Targeted quantitative analysis of Streptococcus pyogenes virulence factors by multiple reaction monitoring. Mol. Cell. Proteomics 7, 1489–1500 (2008).
Picotti, P. et al. A database of mass spectrometric assays for the yeast proteome. Nat. Methods 5, 913–914 (2008).
Fusaro, V.A., Mani, D.R., Mesirov, J.P. & Carr, S.A. Prediction of high-responding peptides for targeted protein assays by mass spectrometry. Nat. Biotechnol. 27, 190–198 (2009).
Sherwood, C. et al. MaRiMba: a software application for spectral library-based MRM transition list assembly. J. Proteome Res. 8, 4396–4405 (2009).
MacLean, B. et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010).
Prakash, A. et al. Expediting the development of targeted SRM assays: using data from shotgun proteomics to automate method development. J. Proteome Res. 8, 2733–2739 (2009).
Abbatiello, S.E., Mani, D.R., Keshishian, H. & Carr, S.A. Automated detection of inaccurate and imprecise transitions in peptide quantification by multiple reaction monitoring mass spectrometry. Clin. Chem. 56, 291–305 (2010).
Stahl-Zeng, J. et al. High sensitivity detection of plasma proteins by multiple reaction monitoring of N-glycosites. Mol. Cell. Proteomics 6, 1809–1817 (2007).
Nesvizhskii, A.I., Keller, A., Kolker, E. & Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 (2003).
Elias, J.E. & Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Kall, L., Canterbury, J.D., Weston, J., Noble, W.S. & MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 (2007).
Reiter, L. et al. Protein identification false discovery rates for very large proteomics data sets generated by tandem mass spectrometry. Mol. Cell. Proteomics 8, 2405–2417 (2009).
Picotti, P. et al. High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat. Methods 7, 43–46 (2010).
Moore, R.E., Young, M.K. & Lee, T.D. Qscore: an algorithm for evaluating SEQUEST database search results. J. Am. Soc. Mass Spectrom. 13, 378–386 (2002).
Sherman, J., McKay, M.J., Ashman, K. & Molloy, M.P. How specific is my SRM?: The issue of precursor and product ion redundancy. Proteomics 9, 1120–1123 (2009).
Choi, H. & Nesvizhskii, A.I. Semisupervised model-based validation of peptide identifications in mass spectrometry-based proteomics. J. Proteome Res. 7, 254–265 (2008).
Hilpert, K., Winkler, D.F. & Hancock, R.E. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat. Protoc. 2, 1333–1349 (2007).
Wenschuh, H. et al. Coherent membrane supports for parallel microsynthesis and screening of bioactive peptides. Biopolymers 55, 188–206 (2000).
Keller, A., Nesvizhskii, A.I., Kolker, E. & Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 (2002).
Kim, S., Gupta, N. & Pevzner, P.A. Spectral probabilities and generating functions of tandem mass spectra: a strike against decoy databases. J. Proteome Res. 7, 3354–3363 (2008).
Ong, S.E. et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 (2002).
Gerber, S.A., Rush, J., Stemman, O., Kirschner, M.W. & Gygi, S.P. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. USA 100, 6940–6945 (2003).
Pedrioli, P.G. et al. A common open representation of mass spectrometry data and its application to proteomics research. Nat. Biotechnol. 22, 1459–1466 (2004).
Keller, A., Eng, J., Zhang, N., Li, X.J. & Aebersold, R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol. Syst. Biol. 1, 2005.0017 (2005).
Storey, J.D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100, 9440–9445 (2003).
R Development Core Team. R: A Language and Environment for Statistical Computing (2008).
Acknowledgements
We thank J. Malmström and M. Jovanovic for providing the samples that were used as background matrix in the gold-standard data set, M. Jovanovic for careful reading of the manuscript, A. Srebniak for help in generating a software package, and H. Wenschuh. We acknowledge M. Claassen for discussions on machine learning. This work was supported by grants from the Forschungskredit of the University of Zurich, University of Zurich Research Priority Program in Systems Biology and Functional Genomics, GEBERT-RÜF Stiftung and Swiss National Science Foundation (grant 31000-10767), with funds from the US National Heart, Lung, and Blood Institute and the US National Institutes of Health (contract N01-HV-28179), and by SystemsX.ch, the Swiss initiative for systems biology.
Author information
Authors and Affiliations
Contributions
L.R., O.R., P.P., M.-Y.B. and R.A. designed the gold-standard data set. P.P. carried out the measurements on the gold-standard data set. L.R., O.R. and R.A. wrote the paper. L.R. and O.R. wrote the software and did the data analysis. L.R. did most of the statistical data analysis. R.H. contributed to the experiment involving the human plasma N-glycopeptide-enriched samples. M.B. contributed to the experiment involving the human u2os cell line. M.O.H. provided critical input on the project. R.A. supervised the project.
Corresponding author
Ethics declarations
Competing interests
O.R. and L.R. are employees of Biognosys AG. This company funded parts of the work.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Table 1, Supplementary Results and Supplementary Note (PDF 7030 kb)
Supplementary Data 1
Table of transitions, table of peak groups, table with identification statistics and classifier of the gold standard data set analysis. The transitions sheet contains the precursor m/z (Q1), fragment ion m/z (Q3), an id that groups the transitions according to precursor (transition group id), an id for the transition (transition id), a string describing the isotopic labeling of the peptide (isotype), the collision energy used (CE), the expected retention time used for scheduled SRM (tR), the expected relative intensity of the fragment ions (relative intensity %), a string indicating whether the transition is a decoy or target (decoy) and an id to group corresponding target and decoy transition groups (target decoy transition group id). The mProphet peak groups sheet contains a row for each peak group. The most important columns are an id for a transition group measurement (transition_group_record), the features used for scoring (all columns starting with main_var or var_), a column indicating the dilution of the synthetic peptides in the specific matrix (dilution), the species used for the background matrix (background), the class of the peak group in terms of identity as determined by the dilution alignment (real_class), a boolean indicating whether the peak group was derived from decoy or target transitions (real_decoy), a boolean indicating whether treated as decoy or target in the mProphet analysis (decoy) and the mProphet discrimination score (d_score). The mProphet all peak groups sheet contains the all peak groups of the analysis, not only the ones that rank highest in one transition group record (peak_group_rank). The mProphet stat sheet relates the mProphet discrimination score (cutoff) to the false discovery rate (FDR) and the sensitivity (sens). The mProphet classifier weight sheet contains the weights that were determined using the semi-supervised learning approach. (XLS 2515 kb)
Supplementary Data 2
Table of transitions, table of peak groups, table with identification statistics and classifier of the human u2os cell line analysis. For a detailed description of the sheets see Supplementary Data 1 legend. (XLS 3791 kb)
Supplementary Data 3
Table of transitions, table of peak groups, table with identification statistics and classifier of the human plasma analysis. For a detailed description of the sheets see Supplementary Data 1 legend. (XLS 1166 kb)
Supplementary Data 4
Table of transitions and peak groups for the measurement of yeast target and decoy transitions in human plasma. The transitions sheet contains target transitions of yeast peptides and corresponding decoy transitions generated by two different decoy transition generation algorithms (ADD_RANDOM and REVERSE_PEP_AND_INCREASE_Q1). The mQuest peak groups sheet contains the data processed with mQuest. The mProphet analysis does result in meaningful results since the data contains no positive target measurements. For a detailed description of the sheets see Supplementary Data 1 legend. (XLS 675 kb)
Rights and permissions
About this article
Cite this article
Reiter, L., Rinner, O., Picotti, P. et al. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat Methods 8, 430–435 (2011). https://doi.org/10.1038/nmeth.1584
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1584
This article is cited by
-
SeFilter-DIA: Squeeze-and-Excitation Network for Filtering High-Confidence Peptides of Data-Independent Acquisition Proteomics
Interdisciplinary Sciences: Computational Life Sciences (2024)
-
Proteomic profiling of protein expression changes after 3 months-exercise in ESRD patients on hemodialysis
BMC Nephrology (2023)
-
Generalized precursor prediction boosts identification rates and accuracy in mass spectrometry based proteomics
Communications Biology (2023)
-
Benchmarking commonly used software suites and analysis workflows for DIA proteomics and phosphoproteomics
Nature Communications (2023)
-
IRAK4 degrader in hidradenitis suppurativa and atopic dermatitis: a phase 1 trial
Nature Medicine (2023)