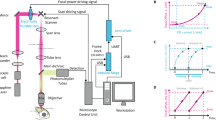
Abstract
Recording electrical activity from identified neurons in intact tissue is key to understanding their role in information processing. Recent fluorescence labeling techniques have opened new possibilities to combine electrophysiological recording with optical detection of individual neurons deep in brain tissue. For this purpose we developed dual-core fiberoptics–based microprobes, with an optical core to locally excite and collect fluorescence, and an electrolyte-filled hollow core for extracellular single unit electrophysiology. This design provides microprobes with tips <10 μm, enabling analyses with single-cell optical resolution. We demonstrate combined electrical and optical detection of single fluorescent neurons in rats and mice. We combined electrical recordings and optical Ca2+ measurements from single thalamic relay neurons in rats, and achieved detection and activation of single channelrhodopsin-expressing neurons in Thy1::ChR2-YFP transgenic mice. The microprobe expands possibilities for in vivo electrophysiological recording, providing parallel access to single-cell optical monitoring and control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
MacLean, J.N., Fenstermaker, V., Watson, B.O. & Yuste, R. A visual thalamocortical slice. Nat. Methods 3, 129–134 (2006).
Swadlow, H.A. Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J. Neurosci. Methods 79, 131–141 (1998).
De Koninck, Y., Ribeiro-da-Silva, A., Henry, J.L. & Cuello, A.C. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. Proc. Natl. Acad. Sci. USA 89, 5073–5077 (1992).
Timofeeva, E., Merette, C., Emond, C., Lavallee, P. & Deschenes, M. A map of angular tuning preference in thalamic barreloids. J. Neurosci. 23, 10717–10723 (2003).
Honig, M.G. & Hume, R.I. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 12, 333–335 (1989).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Spergel, D.J., Kruth, U., Shimshek, D.R., Sprengel, R. & Seeburg, P.H. Using reporter genes to label selected neuronal populations in transgenic mice for gene promoter, anatomical, and physiological studies. Prog. Neurobiol. 63, 673–686 (2001).
Callaway, E.M. Transneuronal circuit tracing with neurotropic viruses. Curr. Opin. Neurobiol. 18, 617–623 (2009).
Luo, L., Callaway, E.M. & Svoboda, K. Genetic dissection of neural circuits. Neuron 57, 634–660 (2008).
Ikeda, H., Heinke, B., Ruscheweyh, R. & Sandkuhler, J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299, 1237–1240 (2003).
Labrakakis, C. & Macdermott, A.B. Neurokinin receptor 1-expressing spinal cord neurons in lamina I and III/IV of postnatal rats receive inputs from capsaicin sensitive fibers. Neurosci. Lett. 352, 121–124 (2003).
Yuste, R. Fluorescence microscopy today. Nat. Methods 2, 902–904 (2005).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Margrie, T.W. et al. Targeted whole-cell recordings in the mammalian brain in vivo. Neuron 39, 911–918 (2003).
Jung, J.C., Mehta, A.D., Aksay, E., Stepnoski, R. & Schnitzer, M.J. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy. J. Neurophysiol. 92, 3121–3133 (2004).
Levene, M.J., Dombeck, D.A., Kasischke, K.A., Molloy, R.P. & Webb, W.W. In vivo multiphoton microscopy of deep brain tissue. J. Neurophysiol. 91, 1908–1912 (2004).
Bowmaster, T.A., Davis, C.C. & Krauthamer, V. Excitation and detection of action potential-induced fluorescence changes through a single monomode optical fiber. Biochim. Biophys. Acta 1091, 9–14 (1991).
Duff Davis, M. & Schmidt, J.J. In vivo spectrometric calcium flux recordings of intrinsic Caudate-Putamen cells and transplanted IMR-32 neuroblastoma cells using miniature fiber optrodes in anesthetized and awake rats and monkeys. J. Neurosci. Methods 99, 9–23 (2000).
Kudo, Y., Takeda, K., Hicks, T.P., Ogura, A. & Kawasaki, Y. A new device for monitoring concentrations of intracellular Ca2+ in CNS preparations and its application to the frog's spinal cord. J. Neurosci. Methods 30, 161–168 (1989).
Nakamura, T. et al. Increased intracellular Ca2+ concentration in the hippocampal CA1 area during global ischemia and reperfusion in the rat: a possible cause of delayed neuronal death. Neuroscience 88, 57–67 (1999).
Bradley, P.M., Murphy, D., Kasparov, S., Croker, J. & Paton, J.F. A micro-optrode for simultaneous extracellular electrical and intracellular optical recording from neurons in an intact oscillatory neuronal network. J. Neurosci. Methods 168, 383–395 (2008).
Humphrey, D.R. & Schmidt, E.M. Extracellular single-unit recording methods. Neuromethods 15, 1–64 (1991).
Bellavance, M.A., Demers, M. & Deschenes, M. Feedforward inhibition determines the angular tuning of vibrissal responses in the principal trigeminal nucleus. J. Neurosci. 30, 1057–1063 (2010).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Wang, H. et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc. Natl. Acad. Sci. USA 104, 8143–8148 (2007).
Gradinaru, V., Mogri, M., Thompson, K.R., Henderson, J.M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009).
Lima, S.Q., Hromadka, T., Znamenskiy, P. & Zador, A.M. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE 4, e6099 (2009).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Bennett, P.J., Monro, T.M. & Richardson, D.J. Toward practical holey fiber technology: fabrication, splicing, modeling, and characterization. Opt. Lett. 24, 1203–1205 (1999).
Knight, J.C., Birks, T.A., Russell, P.S. & Atkin, D.M. All-silica single-mode optical fiber with photonic crystal cladding. Opt. Lett. 21, 1547–1549 (1996).
MacChesney, J.B., O'Connor, P.B. & Presby, H.M. New technique for preparation of low-loss and graded-index optical fibers. Proc. IEEE 62, 1280–1281 (1974).
Lopez-Bendito, G. et al. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb. Cortex 14, 1122–1133 (2004).
Buhl, E.H. & Lubke, J. Intracellular Lucifer Yellow injection in fixed brain slices combined with retrograde tracing, light and electron microscopy. Neuroscience 28, 3–16 (1989).
Acknowledgements
This work was supported by the Canadian Institutes for Health Research. Y.D.K. was funded by a Chercheur National award from the Fonds de la recherche en santé du Québec. Y.L. and S.D. received a Studentship from the Canadian Institutes for Health Research Neurophysics Training program grant. We thank A. Proulx and A. Croteau for the collaboration on the dual-core fiber design and fabrication; D. Côté and A. Castonguay for helpful comments on the manuscript; and S.L. Côté, K. Bachand, K. Vandal and M. Demers for expert technical assistance.
Author information
Authors and Affiliations
Contributions
Y.L., R.V. and Y.D.K. designed the microprobe. Y.L., S.D., G.L., C.B., M.D. and Y.D.K. designed the experiments. Y.L., S.D., G.L. and Y.D.K. analyzed data. Y.L., S.D., G.L. and C.B. performed experiments. S.D. performed Ca2+ measurement and photostimulation experiments. Y.L. and S.D. performed numerical simulations. Y.L., S.D., G.L., C.B., M.D., R.V. and Y.D.K. contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Data (PDF 1592 kb)
Rights and permissions
About this article
Cite this article
LeChasseur, Y., Dufour, S., Lavertu, G. et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat Methods 8, 319–325 (2011). https://doi.org/10.1038/nmeth.1572
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1572
This article is cited by
-
Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits
Nature Biotechnology (2023)
-
Discrete hippocampal projections are differentially regulated by parvalbumin and somatostatin interneurons
Nature Communications (2023)
-
Recent developments in multifunctional neural probes for simultaneous neural recording and modulation
Microsystems & Nanoengineering (2023)
-
All-optical interrogation of neural circuits in behaving mice
Nature Protocols (2022)
-
Tapered fibertrodes for optoelectrical neural interfacing in small brain volumes with reduced artefacts
Nature Materials (2022)