Abstract
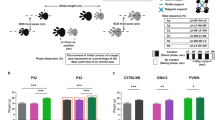
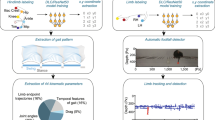
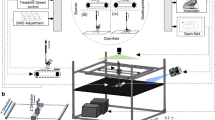
Rodents are frequently used to model damage and diseases of the central nervous system (CNS) that lead to functional deficits. Impaired locomotor function is currently evaluated by using scoring systems or biomechanical measures. These methods often suffer from limitations such as subjectivity, nonlinearity and low sensitivity, or focus on a few very restricted aspects of movement. Thus, full quantitative profiles of motor deficits after CNS damage are lacking. Here we report the detailed characterization of locomotor impairments after applying common forms of CNS damage in rodents. We obtained many objective and quantitative readouts from rats with either spinal cord injuries or strokes and from transgenic mice (Epha4−/−) during skilled walking, overground walking, wading and swimming, resulting in model-specific locomotor profiles. Our testing and analysis method enables comprehensive assessment of locomotor function in rodents and has broad application in various fields of life science research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Basso, D.M. Neuroanatomical substrates of functional recovery after experimental spinal cord injury: implications of basic science research for human spinal cord injury. Phys. Ther. 80, 808–817 (2000).
Raineteau, O. & Schwab, M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 (2001).
McEwen, M.L. & Springer, J.E. Quantification of locomotor recovery following spinal cord contusion in adult rats. J. Neurotrauma 23, 1632–1653 (2006).
Muir, G.D. & Webb, A.A. Mini-review: assessment of behavioural recovery following spinal cord injury in rats. Eur. J. Neurosci. 12, 3079–3086 (2000).
Metz, G.A., Merkler, D., Dietz, V., Schwab, M.E. & Fouad, K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 883, 165–177 (2000).
Kunkel-Bagden, E., Dai, H.N. & Bregman, B.S. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp. Neurol. 119, 153–164 (1993).
Basso, D.M., Beattie, M.S. & Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 (1995).
Hamers, F.P., Lankhorst, A.J., van Laar, T.J., Veldhuis, W.B. & Gispen, W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma 18, 187–201 (2001).
Courtine, G. et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 12, 1333–1342 (2009).
Magnuson, D.S. et al. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil. Neural Repair 23, 535–545 (2009).
Gorska, T., Chojnicka-Gittins, B., Majczynski, H. & Zmyslowski, W. Recovery of overground locomotion following partial spinal lesions of different extent in the rat. Behav. Brain Res. 196, 286–296 (2009).
Basso, D.M. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma 21, 395–404 (2004).
Roy, R.R., Hutchison, D.L., Pierotti, D.J., Hodgson, J.A. & Edgerton, V.R. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol. 70, 2522–2529 (1991).
Bolton, D.A., Tse, A.D., Ballermann, M., Misiaszek, J.E. & Fouad, K. Task specific adaptations in rat locomotion: runway versus horizontal ladder. Behav. Brain Res. 168, 272–279 (2006).
Garnier, C., Falempin, M. & Canu, M.H. A 3D analysis of fore- and hindlimb motion during locomotion: comparison of overground and ladder walking in rats. Behav. Brain Res. 186, 57–65 (2008).
Kanagal, S.G. & Muir, G.D. Task-dependent compensation after pyramidal tract and dorsolateral spinal lesions in rats. Exp. Neurol. 216, 193–206 (2009).
Metz, G.A. & Whishaw, I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods 115, 169–179 (2002).
Dottori, M. et al. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc. Natl. Acad. Sci. USA 95, 13248–13253 (1998).
Gruner, J.A. & Altman, J. Swimming in the rat: analysis of locomotor performance in comparison to stepping. Exp. Brain Res. 40, 374–382 (1980).
Schapiro, S., Salas, M. & Vukovich, K. Hormonal effects on ontogeny of swimming ability in the rat: assessment of central nervous system development. Science 168, 147–150 (1970).
Liebscher, T. et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 58, 706–719 (2005).
Kiehn, O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306 (2006).
Goulding, M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518 (2009).
Juvin, L., Simmers, J. & Morin, D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J. Neurosci. 25, 6025–6035 (2005).
Grillner, S., Wallen, P., Saitoh, K., Kozlov, A. & Robertson, B. Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res. Brain Res. Rev. 57, 2–12 (2008).
Hagglund, M., Borgius, L., Dougherty, K.J. & Kiehn, O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat. Neurosci. 13, 246–252 (2010).
Frigon, A. & Rossignol, S. Experiments and models of sensorimotor interactions during locomotion. Biol. Cybern. 95, 607–627 (2006).
Butt, S.J., Lundfald, L. & Kiehn, O. EphA4 defines a class of excitatory locomotor-related interneurons. Proc. Natl. Acad. Sci. USA 102, 14098–14103 (2005).
Yamaguchi, T. The central pattern generator for forelimb locomotion in the cat. Prog. Brain Res. 143, 115–122 (2004).
Ghosh, A. et al. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 29, 12210–12219 (2009).
Schucht, P., Raineteau, O., Schwab, M.E. & Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 (2002).
Kaegi, S., Schwab, M.E., Dietz, V. & Fouad, K. Electromyographic activity associated with spontaneous functional recovery after spinal cord injury in rats. Eur. J. Neurosci. 16, 249–258 (2002).
Fischer, M.S., Schilling, N., Schmidt, M., Haarhaus, D. & Witte, H. Basic limb kinematics of small therian mammals. J. Exp. Biol. 205, 1315–1338 (2002).
Filipe, V.M. et al. Effect of skin movement on the analysis of hindlimb kinematics during treadmill locomotion in rats. J. Neurosci. Methods 153, 55–61 (2006).
Kloos, A.D., Fisher, L.C., Detloff, M.R., Hassenzahl, D.L. & Basso, D.M. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp. Neurol. 191, 251–265 (2005).
Acknowledgements
We dedicate this work to the memory of our late colleague and friend Eva Hochreutener, who contributed excellent graphical work and suggestions essential to this study. We thank R. Schöb and P. Scheuble for IT support and S. Giger for construction of the behavioral testing system, L. Schnell for her pioneering work on behavior testing and evaluation in our laboratory, M. Petrinovic for help with mouse testing, R. Klein (Department of Molecular Neurobiology, Max Planck Institute of Neurobiology, Martinsried) for providing the Epha4−/− mice and members of the software company ALEA Solutions GmbH for close collaboration and for the development of the tracking software. This study was supported by grants of the Swiss National Science Foundation (31-63633.00 and 3100AO-122527/1), the National Centre for Competence in Research 'Neural Plasticity and Repair' of the Swiss National Science Foundation, the Spinal Cord Consortium of the Christopher and Dana Reeve Foundation and the Framework Program 7 EU Collaborative Project Spinal Cord Repair.
Author information
Authors and Affiliations
Contributions
B.Z. and L.F. designed the study, developed the testing setup, performed surgeries, collected and analyzed data, made the figures and prepared the manuscript. M.L.S. developed stroke lesions, performed surgeries and prepared the manuscript. R.G. developed the EMG setup, performed the recordings and collected and analyzed data. H.K. developed the testing setup and developed software. M.R. performed surgeries and collected and analyzed data. M.B. developed software and collected and analyzed data. M.E.S. designed the study, prepared the manuscript, and conceived and supervised the study.
Corresponding author
Ethics declarations
Competing interests
B.Z., L.F., M.L.S., H.K. and M.E.S. consult with the company TSE Systems GmbH for the development of a commercially available version of the rodent testing setup.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Note 1 (PDF 140 kb)
Rights and permissions
About this article
Cite this article
Zörner, B., Filli, L., Starkey, M. et al. Profiling locomotor recovery: comprehensive quantification of impairments after CNS damage in rodents. Nat Methods 7, 701–708 (2010). https://doi.org/10.1038/nmeth.1484
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1484
This article is cited by
-
Evaluation of the Effectiveness of Systemic Therapy of Spinal Cord Injury of Moderate Severity with Human Umbilical Cord Placental Blood Mononuclear Cells Using Indicators of Dispersion of Articular Angles in the Swimming Test
Bulletin of Experimental Biology and Medicine (2023)
-
Rodent Models of Spinal Cord Injury: From Pathology to Application
Neurochemical Research (2023)
-
Deep learning-based behavioral profiling of rodent stroke recovery
BMC Biology (2022)
-
Targeted activation of midbrain neurons restores locomotor function in mouse models of parkinsonism
Nature Communications (2022)
-
Progression in translational research on spinal cord injury based on microenvironment imbalance
Bone Research (2022)