Abstract
Optical microscopy has been a fundamental tool of biological discovery for more than three centuries, but its in vivo tissue imaging ability has been restricted by light scattering to superficial investigations, even when confocal or multiphoton methods are used. Recent advances in optical and optoacoustic (photoacoustic) imaging now allow imaging at depths and resolutions unprecedented for optical methods. These abilities are increasingly important to understand the dynamic interactions of cellular processes at different systems levels, a major challenge of postgenome biology. This Review discusses promising photonic methods that have the ability to visualize cellular and subcellular components in tissues across different penetration scales. The methods are classified into microscopic, mesoscopic and macroscopic approaches, according to the tissue depth at which they operate. Key characteristics associated with different imaging implementations are described and the potential of these technologies in biological applications is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Beauvoit, B., Evans, S.M., Jenkins, T.W., Miller, E.E. & Chance, B. Correlation between the light-scattering and the mitochondrial content of normal-tissues and transplantable rodent tumors. Anal. Biochem. 226, 167–174 (1995).
Webb, R.H. Theoretical basis of confocal microscopy. Methods Enzymol. 307, 3–20 (1999).A concise description of confocal microscopy technology and performance metrics.
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).An introduction of two-photon laser scanning fluorescence microscopy.
Helmchen, F. & Denk, W. Deep-tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Tsien, R.Y. Building and breeding molecules to spy on cells and tumors. FEBS Lett. 579, 927–932 (2005).A concise review of fluorescence reporters and probes for in vivo imaging.
Weissleder, R. & Pittet, M. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Stephens, D.J. & Allan, V.J. Light microscopy techniques for live cell imaging. Science 300, 82–86 (2003).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1368–1376 (2003).
Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Sharpe, J. et al. Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296, 541–545 (2002).An introduction of optical projection tomography.
Walls, J.R., Sled, J.G., Sharpe, J. & Henkelman, R.M. Resolution improvement in emission optical projection tomography. Phys. Med. Biol. 52, 2775–2790 (2007).
Alanentalo, T. et al. High-resolution three-dimensional imaging of islet-infiltrate interactions based on optical projection tomography assessments of the intact adult mouse pancreas. J. Biomed. Opt. 13, 054070 (2008).
Kerwin, J. et al. 3 dimensional modelling of early human brain development using optical projection tomography. BMC Neurosci. 5, 27 (2004).
Boot, M.J. et al. In vitro whole-organ imaging: 4D quantification of growing mouse limb buds. Nat. Methods 5, 609–612 (2008).
Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J. & Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004).An introduction of selective plane illumination microscopy.
Huisken, J. & Stainier, D.Y. Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM). Opt. Lett. 32, 2608–2610 (2007).
Verveer, P.J. et al. High-resolution three-dimensional imaging of large specimens with light sheet-based microscopy. Nat. Methods 4, 311–313 (2007).
Dodt, H.U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007).
Ermolayev, V. et al. Ultramicroscopy reveals axonal transport impairments in cortical motor neurons at prion disease. Biophys. J. 96, 3390–3398 (2009).
Andreev, V.G., Karabutov, A.A. & Oraevsky, A.A. Detection of ultrawide-band ultrasound pulses in optoacoustic tomography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 50, 1383–1390 (2003).
Wang, X. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 21, 803–806 (2003).A demonstration of blood-vessel imaging using optoacoustic (photoacoustic) tomography.
Ntziachristos, V., Ripoll, J., Wang, L.H.V. & Weissleder, R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat. Biotechnol. 23, 313–320 (2005).
Zhang, H., Maslov, K., Stoica, G. & Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 24, 848–851 (2006).An introduction of functional photoacoustic microscopy.
Razansky, D., Vinegoni, C. & Ntziachristos, V. Multispectral photoacoustic imaging of fluorochromes in small animals. Opt. Lett. 32, 2891–2893 (2007).
Maslov, K., Stoica, G. & Wang, L. In vivo dark field reflection-mode photoacoustic microscopy. Opt. Lett. 30, 625–627 (2005).
Maslov, K., Zhang, H., Hu, S. & Wang, L. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 33, 929–931 (2008).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Tearney, G.J. et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science 276, 2037–2039 (1997).
Bredfeldt, J.S., Vinegoni, C., Marks, D.L. & Boppart, S.A. Molecularly sensitive optical coherence tomography. Opt. Lett. 30, 495–497 (2005).
Skala, M.C., Crow, M.J., Wax, A. & Izatt, J.A. Photothermal optical coherence tomography of epidermal growth factor receptor in live cells using immunotargeted gold nanospheres. Nano Lett. 8, 3461–3467 (2008).
Sarunic, M.V., Applegate, B.E. & Izatt, J.A. Spectral domain second-harmonic optical coherence tomography. Opt. Lett. 30, 2391–2393 (2005).
Drexler, W. & Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 27, 45–88 (2008).
Chamberland, D. et al. Photoacoustic tomography of joints aided by an Etanercept-conjugated gold nanoparticle contrast agent—an ex vivo preliminary rat study. Nanotechnology 19, 095101 (2008).
Tolentino, T.P. et al. Measuring diffusion and binding kinetics by contact area FRAP. Biophys. J. 95, 920–930 (2008).
McNally, J.G. Quantitative FRAP in analysis of molecular binding dynamics in vivo. Methods Cell Biol. 85, 329–351 (2008).
Mavrakis, M., Rikhy, R., Lilly, M. & Lippincott-Schwartz, J. Fluorescence imaging techniques for studying Drosophila embryo development. Curr. Protoc. Cell Biol. 4, 18 (2008).
Sprague, B.L., Pego, R.L., Stavreva, D.A. & McNally, J.G. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 86, 3473–3495 (2004).
Provenzano, P.P., Eliceiri, K.W. & Keely, P.J. Multiphoton microscopy and fluorescence lifetime imaging microscopy (FLIM) to monitor metastasis and the tumor microenvironment. Clin. Exp. Metastasis 26, 357–370 (2008).
Hallworth, R., Currall, B., Nichols, M.G., Wu, X. & Zuo, J. Studying inner ear protein-protein interactions using FRET and FLIM. Brain Res. 1091, 122–131 (2006).
Chen, Y., Mills, J.D. & Periasamy, A. Protein localization in living cells and tissues using FRET and FLIM. Differentiation 71, 528–541 (2003).
Tadrous, P.J. Methods for imaging the structure and function of living tissues and cells: 2. fluorescence lifetime imaging. J. Pathol. 191, 229–234 (2000).
Bastiaens, P.I. & Squire, A. Fluorescence lifetime imaging microscopy: spatial resolution of biochemical processes in the cell. Trends Cell Biol. 9, 48–52 (1999).
Rodriguez, L.G., Lockett, S.J. & Holtom, G.R. Coherent anti-stokes Raman scattering microscopy: a biological review. Cytometry A 69, 779–791 (2006).
Rinia, H.A., Wurpel, G.W. & Muller, M. Measuring molecular order and orientation using coherent anti-stokes Raman scattering microscopy. Methods Mol. Biol. 400, 45–61 (2007).
Cheng, J.X. Coherent anti-Stokes Raman scattering microscopy. Appl. Spectrosc. 61, 197–208 (2007).
Imanishi, Y., Lodowski, K.H. & Koutalos, Y. Two-photon microscopy: shedding light on the chemistry of vision. Biochemistry 46, 9674–9684 (2007).
Botvinick, E.L. & Shah, J.V. Laser-based measurements in cell biology. Methods Cell Biol. 82, 81–109 (2007).
Campagnola, P.J. & Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 21, 1356–1360 (2003).
Patterson, M.S., Chance, B. & Wilson, B.C. Time resolved reflectance and transmittance for the noninvasive measurement of tissue optical-properties. Appl. Opt. 28, 2331–2336 (1989).
Arridge, S.R. Optical tomography in medical imaging. Inverse Probl. 15, R41–R93 (1999).
Shu, X. et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science 324, 804–807 (2009).
Shashkov, E., Everts, M., Galanzha, E. & Zharov, V. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 8, 3953–3958 (2008).
De La Zerda et al. Carbon nano-tubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 3, 557–562 (2008).
Weissleder, R. & Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 9, 123–128 (2003).
Ntziachristos, V., Tung, C.H., Bremer, C. & Weissleder, R. Fluorescence molecular tomography resolves protease activity in vivo. Nat. Med. 8, 757–760 (2002).An introduction of fluorescence molecular tomography.
Ntziachristos, V. & Weissleder, R. Experimental three-dimensional fluorescence reconstruction of diffuse media using a normalized Born approximation. Opt. Lett. 26, 893–895 (2001).
Schwaiger, M., Ziegler, S. & Nekolla, S. PET/CT: challenge for nuclear cardiology. J. Nucl. Med. 46, 1664–1678 (2005).
Judenhofer, M.S. et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med. 14, 459–465 (2008).
Barbour, R. et al. MRI-guided optical tomography: prospects and computation for a new imaging method. IEEE Comput. Sci. Eng. 2, 63–77 (1995).
Ntziachristos, V., Yodh, A.G., Schnall, M. & Chance, B. Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc. Natl. Acad. Sci. USA 97, 2767–2772 (2000).
Brooksby, B. et al. Imaging breast adipose and fibroglandular tissue molecular signatures by using hybrid MRI-guided near-infrared spectral tomography. Proc. Natl. Acad. Sci. USA 103, 8828–8833 (2006).
Davis, S.C. et al. Magnetic resonance-coupled fluorescence tomography scanner for molecular imaging of tissue. Rev. Sci. Instrum. 79, 064302 (2008).
Fang, Q. et al. Combined optical imaging and mammography of the healthy breast: optical contrast derived from breast structure and compression. IEEE Trans. Med. Imaging 28, 30–42 (2009).
Schulz, R. et al. Hybrid system for simultaneous fluorescence and X-ray computed tomography. IEEE Trans. Med. Imaging 29, 465–473 (2010).
Hyde, D. et al. Hybrid FMT-CT imaging of amyloid-beta plaques in a murine Alzheimer's disease model. Neuroimage 44, 1304–1311 (2009).
Lin, Y., Gao, H., Nalcioglu, O. & Gulsen, G. Fluorescence diffuse optical tomography with functional and anatomical a priori information: feasibility study. Phys. Med. Biol. 52, 5569–5585 (2007).
Guven, M., Yazici, B., Intes, X. & Chance, B. Diffuse optical tomography with a priori anatomical information. Phys. Med. Biol. 50, 2837–2858 (2005).
Hintersteiner, M. et al. In vivo detection of amyloid-beta deposits by near-infrared imaging using an oxazine-derivative probe. Nat. Biotechnol. 23, 577–583 (2005).
Cox, B.T., Arridge, S.R., Kostli, K.P. & Beard, P.C. Two-dimensional quantitative photoacoustic image reconstruction of absorption distributions in scattering media by use of a simple iterative method. Appl. Opt. 45, 1866–1875 (2006).
Rosenthal, A., Razansky, D. & Ntziachristos, V. Fast semi-analytical model-based acoustic inversion for quantitative optoacoustic tomography. IEEE Trans. Med. Imaging 29, 1275–1285 (2010).
Bowen, T. Radiation-induced thermoacoustic soft-tissue imaging. Proc. IEEE Ultrason. Symp. 817–822 (1981).
Zemp, R.J. et al. Photoacoustic imaging of the microvasculature with a high-frequency ultrasound array transducer. J. Biomed. Opt. 12, 010501 (2007).
Allen, T.J. & Beard, P.C. Pulsed near-infrared laser diode excitation system for biomedical photoacoustic imaging. Opt. Lett. 31, 3462–3464 (2006).
Kolkman, R.G.M. et al. Photoacoustic determination of blood vessel diameter. Phys. Med. Biol. 49, 4745–4756 (2004).
Eghtedari, M. et al. High sensitivity of in vivo detection of gold nanorods using a laser optoacoustic imaging system. Nano Lett. 7, 1914–1918 (2007).
Cox, B., Arridge, S. & Beard, P. Estimating chromophore distributions from multiwavelength photoacoustic images. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 26, 443–455 (2009).
Jetzfellner, T., Rozenthal, A., Englmeier, K., Razansky, D. & Ntziachristos, V. Multispectral optoacoustic tomography by means of normalized spectral ratio. Opt. Lett. (in the press).
Li, M. et al. Simultaneous molecular and hypoxia imaging of brain tumors in vivo using spectroscopic photoacoustic tomography. Proc. IEEE 96, 481–489 (2008).
Li, L., Zemp, R., Lungu, G., Stoica, G. & Wang, L. Photoacoustic imaging of lacZ gene expression in vivo. J. Biomed. Opt. 12, 020504 (2007).
Kruger, R.A, Kiser, W. Jr., Reinecke, D., Kruger, G. & Miller, K. Thermoacoustic optical molecular imaging of small animals. Mol. Imaging 2, 113–123 (2003).
Rayavarapu, R. et al. Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques. Int. J. Biomed. Imaging 2007 29817 (2007).
Li, L., Zemp, R.J., Lungu, G., Stoica, G. & Wang, L.V. Photoacoustic imaging of lacZ gene expression in vivo. J. Biomed. Opt. 12, 020504 (2007).
Galanzha, E. et al. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat. Nanotechnol. 4, 855–860 (2009).
Vinegoni, C., Pitsouli, C., Razansky, D., Perrimon, N. & Ntziachristos, V. In vivo imaging of Drosophila melanogaster pupae with mesoscopic fluorescence tomography. Nat. Methods 5, 45–47 (2008).
Razansky, D. et al. Imaging of mesoscopic targets using selective-plane optoacoustic tomography. Nat. Photonics 3, 412–417 (2009).A demonstration of visualizing optical reporter molecules in vivo using mesoscopic multispectral optoacoustic tomography.
Jain, R.K., Munn, L.L. & Fukumura, D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer 2, 266–276 (2002).A description of in vivo applications of intravital microscopy in cancer.
Wang, T.D., Contag, C.H., Mandella, M.J., Chan, N.Y. & Kino, G.S. Confocal fluorescence microscope with dual-axis architecture and biaxial postobjective scanning. J. Biomed. Opt. 9, 735–742 (2004).
Sipkins, D.A. et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005).
Kleinfeld, D., Mitra, P.P., Helmchen, F. & Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl. Acad. Sci. USA 95, 15741–15746 (1998).
Majewska, A.K., Newton, J.R. & Sur, M. Remodeling of synaptic structure in sensory cortical areas in vivo. J. Neurosci. 26, 3021–3029 (2006).
Germain, R.N. et al. An extended vision for dynamic high-resolution intravital immune imaging. Semin. Immunol. 17, 431–441 (2005).
Molitoris, B.A. & Sandoval, R.M. Intravital multiphoton microscopy of dynamic renal processes. Am. J. Physiol. Renal Physiol. 288, F1084–F1089 (2005).
Jobsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 (1977).
Boas, D.A., Oleary, M.A., Chance, B. & Yodh, A.G. Scattering of diffuse photon fensity eaves ny dpherical inhomogeneities within turbid media—analytic solution and applications. Proc. Natl. Acad. Sci. USA 91, 4887–4891 (1994).
Schotland, J.C. Continuous-wave diffusion imaging. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 14, 275–279 (1997).
Cheong, W., Prahl, S. & Welch, A. A review of the optical-properties of biological tissues. IEEE J. Quantum Electron. 26, 2166–2185 (1990).
Beek, J.F., van Staveren, H.J., Posthumus, P., Sterenborg, H.J.C.M. & van Gemert, M.J.C. The optical properties of lung as a function of respiration. Phys. Med. Biol. 42, 2263–2272 (1997).
Pogue, B.W. et al. Characterization of hemoglobin, water, and NIR scattering in breast tissue: analysis of intersubject variability and menstrual cycle changes. J. Biomed. Opt. 9, 541–552 (2004).
Niedre, M., Turner, G. & Ntziachristos, V. Time-resolved imaging of optical coefficients through murine chest cavities. J. Biomed. Opt. 11, 064017–064011–064017 (2006).
Acknowledgements
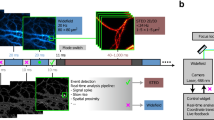
I acknowledge investigators and students for their multiple contributions in understanding optical and optoacoustic performance; J. Ripoll for the contribution of Figure 1b; and support from a European Research Council Senior Investigator Award, the German Federal Ministry of Education and Research and the Institute for Biological and Medical Imaging
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods 7, 603–614 (2010). https://doi.org/10.1038/nmeth.1483
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1483
This article is cited by
-
Engineered serum markers for non-invasive monitoring of gene expression in the brain
Nature Biotechnology (2024)
-
Revolutionizing medical imaging: a comprehensive review of optical coherence tomography (OCT)
Journal of Optics (2024)
-
Functional photoacoustic imaging: from nano- and micro- to macro-scale
Nano Convergence (2023)
-
Phase conjugation with spatially incoherent light in complex media
Nature Photonics (2023)
-
All-optical image classification through unknown random diffusers using a single-pixel diffractive network
Light: Science & Applications (2023)