Abstract
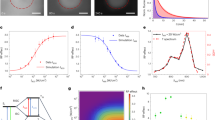
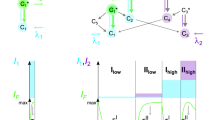
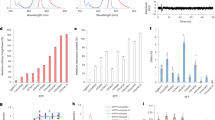
We found that photoconversion is fairly common among orange and red fluorescent proteins, as in a screen of 12 proteins, 8 exhibited photoconversion. Specifically, three red fluorescent proteins could be switched to a green state, and two orange variants could be photoconverted to a far-red state. The orange proteins are ideal for dual-probe highlighter applications, and they exhibited the most red-shifted excitation of all fluorescent proteins described to date.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Patterson, G.H. & Lippincott-Schwartz, J. Science 297, 1873–1877 (2002).
Chudakov, D.M. et al. Nat. Biotechnol. 22, 1435–1439 (2004).
Gurskaya, N.G. et al. Nat. Biotechnol. 24, 461–465 (2006).
Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Nat. Protocols 2, 2024–2032 (2007).
Wiedenmann, J. et al. Proc. Natl. Acad. Sci. USA 101, 15905–15910 (2004).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Tsutsui, H., Karasawa, S., Shimizu, H., Nukina, N. & Miyawaki, A. EMBO Rep. 6, 233–238 (2005).
Shcherbo, D. et al. Nat. Methods 4, 741–746 (2007).
Merzlyak, E.M. et al. Nat. Methods 4, 555–557 (2007).
Shaner, N.C. et al. Nat. Methods 5, 545–551 (2008).
Gurskaya, N.G. et al. FEBS Lett. 507, 16–20 (2001).
Shaner, N.C. et al. Nat. Biotechnol. 22, 1567–1572 (2004).
Karasawa, S., Araki, T., Nagai, T., Mizuno, H. & Miyawaki, A. Biochem. J. 381, 307–312 (2004).
Goedhart, J., Vermeer, J.E., Adjobo-Hermans, M.J., van Weeren, L. & Gadella, T.W. Jr. PLoS ONE 2, e1011 (2007).
Dittrich, P.S., Schafer, S.P. & Schwille, P. Biophys. J. 89, 3446–3455 (2005).
Habuchi, S. et al. Proc. Natl. Acad. Sci. USA 102, 9511–9516 (2005).
Marchant, J.S., Stutzmann, G.E., Leissring, M.A., LaFerla, F.M. & Parker, I. Nat. Biotechnol. 19, 645–649 (2001).
Habuchi, S. et al. J. Am. Chem. Soc. 127, 8977–8984 (2005).
Valentin, G. et al. Nat. Methods 2, 801 (2005).
Betzig, E. et al. Science 313, 1642–1645 (2006).
Acknowledgements
We thank D.M. Chudakov (Shemiakin-Ovchinnikov Institute of Bioorganic Chemistry,), A. Miyawaki (RIKEN Brain Science Institute) and R.Y. Tsien (University of California at San Diego) for providing plasmids encoding fluorescent protein variants. Measurements were performed in part at the Vanderbilt University Medical Center Cell Imaging Shared Resource (National Institutes of Health grants CA68485, DK20593, DK58404) and at the Quantitative Fluorescence Microcopy course at Mount Desert Island Biological Lab. We thank G. Daniels (Leica), J. Wailes (Zeiss), B. Burklow (Olympus) and S. Schwartz (Nikon) for assistance with their microscopes, and B. Livesay for help with the mVenus experiments. This work was supported by National Institutes of Health grant GM72048 (to D.W.P.).
Author information
Authors and Affiliations
Contributions
G.J.K. designed the experiments, performed part of the microscopy experiments, analyzed and organized all data collected by other authors, and prepared the manuscript; K.L.H. and C.S.M. constructed mammalian expression vectors and performed part of the microscopy experiments; M.W.D. constructed mammalian expression vectors and contributed to editing the manuscript; D.W.P. contributed to the conceptual design and manuscript preparation.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-4 and Supplementary Notes 1-2 (PDF 1688 kb)
Supplementary Video 1
Optical highlighting with mOrange2 during mitosis. Time-lapse imaging of a HeLa cell expressing H2B-mOrange2 going through mitosis after part of the nucleus was photoconverted during prophase. (MOV 1221 kb)
Rights and permissions
About this article
Cite this article
Kremers, GJ., Hazelwood, K., Murphy, C. et al. Photoconversion in orange and red fluorescent proteins. Nat Methods 6, 355–358 (2009). https://doi.org/10.1038/nmeth.1319
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1319
This article is cited by
-
A fluorescence-based binding assay for proteins using the cell surface as a sensing platform
Analytical Sciences (2024)
-
Architecture and dynamics of a desmosome–endoplasmic reticulum complex
Nature Cell Biology (2023)
-
Chromophore reduction plus reversible photobleaching: how the mKate2 “photoconversion” works
Photochemical & Photobiological Sciences (2021)
-
Skin and gut imprinted helper T cell subsets exhibit distinct functional phenotypes in central nervous system autoimmunity
Nature Immunology (2021)
-
In vivo characterisation of fluorescent proteins in budding yeast
Scientific Reports (2019)