Abstract
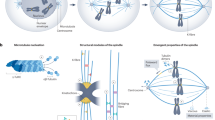
Accurate chromosome segregation during meiosis depends on the assembly of a microtubule-based spindle of proper shape and size. Current models for spindle-size control focus on reaction diffusion–based chemical regulation and balance in activities of motor proteins. Although several molecular perturbations have been used to test these models, controlled mechanical perturbations have not been possible. Here we report a piezoresistive dual cantilever–based system to test models for spindle-size control and examine the mechanical features, such as deformability and stiffness, of the vertebrate meiotic spindle. We found that meiotic spindles prepared in Xenopus laevis egg extracts were viscoelastic and recovered their original shape in response to small compression. Larger compression resulted in plastic deformation, but the spindle adapted to this change, establishing a stable mechanical architecture at different sizes. The technique we describe here may also be useful for examining the micromechanics of other cellular organelles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goshima, G., Wollman, R., Stuurman, N., Scholey, J.M. & Vale, R.D. Length control of the metaphase spindle. Curr. Biol. 15, 1979–1988 (2005).
Gadde, S. & Heald, R. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 14, R797–R805 (2004).
Mitchison, T.J. & Salmon, E.D. Mitosis: a history of division. Nat. Cell Biol. 3, E17–E21 (2001).
Inoue, S. & Salmon, E.D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell 6, 1619–1640 (1995).
Nicklas, R.B. Measurements of the force produced by the mitotic spindle in anaphase. J. Cell Biol. 97, 542–548 (1983).
Inoue, S., Fuseler, J., Salmon, E.D. & Ellis, G.W. Functional organization of mitotic microtubules. Physical chemistry of the in vivo equilibrium system. Biophys. J. 15, 725–744 (1975).
Valentine, M.T., Perlman, Z.E., Mitchison, T.J. & Weitz, D.A. Mechanical properties of Xenopus egg cytoplasmic extracts. Biophys. J. 88, 680–689 (2005).
Onoe, H., Gel, M., Hoshino, K., Matsumoto, K. & Shimoyama, I. Direct measurement of the binding force between microfabricated particles and a planar surface in aqueous solution by force-sensing piezoresistive cantilevers. Langmuir 21, 11251–11261 (2005).
Nagayama, M., Haga, H., Takahashi, M., Saitoh, T. & Kawabata, K. Contribution of cellular contractility to spatial and temporal variations in cellular stiffness. Exp. Cell Res. 300, 396–405 (2004).
Marshall, W.F. Cellular length control systems. Annu. Rev. Cell Dev. Biol. 20, 677–693 (2004).
Varga, V. et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat. Cell Biol. 8, 957–962 (2006).
Hildebrandt, E.R. & Hoyt, M.A. Mitotic motors in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1496, 99–116 (2000).
Kapitein, L.C. et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it cross-links. Nature 435, 114–118 (2005).
Valentine, M.T., Fordyce, P.M., Krzysiak, T.C., Gilbert, S.P. & Block, S.M. Individual dimers of the mitotic kinesin motor Eg5 step processively and support substantial loads in vitro. Nat. Cell Biol. 8, 470–476 (2006).
Dogterom, M., Kerssemakers, J.W., Romet-Lemonne, G. & Janson, M.E. Force generation by dynamic microtubules. Curr. Opin. Cell Biol. 17, 67–74 (2005).
Mallik, R., Carter, B.C., Lex, S.A., King, S.J. & Gross, S.P. Cytoplasmic dynein functions as a gear in response to load. Nature 427, 649–652 (2004).
Desai, A., Murray, A., Mitchison, T.J. & Walczak, C.E. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385–412 (1999).
Hyman, A. et al. Preparation of modified tubulins. Methods Enzymol. 196, 478–485 (1991).
Tirnauer, J.S., Salmon, E.D. & Mitchison, T.J. Microtubule plus-end dynamics in Xenopus egg extract spindles. Mol. Biol. Cell 15, 1776–1784 (2004).
Acknowledgements
This work was supported in part by Grant-in-Aid for Young Scientists (B) and Grant-in-Aid for Scientific Research on Priority Areas (T.I.), and Grant-in-Aid for Scientific Research (A), The 21st Century Center Of Excellence program and “Establishment of Consolidated Research Institute for Advanced Science and Medical Care” from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S.I.), and a Research Grant from the Human Frontier Science Program (S.I. and T.M.K.). T.M.K. also acknowledges support from National Institutes of Health–National Institute of General Medical Sciences (GM65933).
Author information
Authors and Affiliations
Contributions
T.I. performed the experiments and data analysis. J.T. provided considerable experimental assistance. T.I., T.M.K. and S.I. wrote the manuscript. H.O., K.K. and I.S. contributed to design and provide the piezo-resistive cantilevers. J.G. and Y.S. contributed to the initial project planning and experiment design. All authors discussed the results and commented on the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 613 kb)
Supplementary Video 1
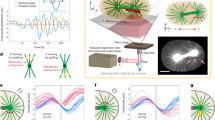
Time-lapse observation of fluorescent meiotic spindle during small deformation. The meiotic spindle, which was sandwiched between the manipulating (left) and the force-sensing (right) cantilevers, was compressed perpendicular to the pole-to-pole axis by moving the manipulating cantilever horizontally with an amplitude of 2 μm on this image plane. Images were acquired every 1 s. Force-dependent deformability and the force response of the spindle are shown in Fig. 2 and Supplementary Fig. 1. Scale bar is 10 μm. (MOV 706 kb)
Supplementary Video 2
Time-lapse observation of fluorescent meiotic spindle during pole-to-pole compression. The meiotic spindle, which was sandwiched between the manipulating (left) and the force-sensing (right) cantilevers, was compressed to the pole-to-pole axis by moving the manipulating cantilever horizontally with an amplitude of 2 to 10-μm range in periodic cycles. Images were acquired every 1 s. Force-dependent deformability and the force response of the spindle at an amplitude of 2 μm are shown in Fig. 3. Scale bar is 10 μm. (MOV 4517 kb)
Supplementary Video 3
Time-lapse observation of fluorescent meiotic spindle during large deformation. The meiotic spindle, which was sandwiched between the manipulating (left) and the force-sensing (right) cantilevers, was compressed perpendicular to the pole-to-pole axis by moving the manipulating cantilever horizontally with an amplitude of 8 to 12-μm range in successive cycles. Images were acquired every 1 s. Force-dependent deformability and the force response of the spindle are shown in Fig. 4 and Supplementary Fig. 3. Scale bar is 10 μm. (MOV 4850 kb)
Rights and permissions
About this article
Cite this article
Itabashi, T., Takagi, J., Shimamoto, Y. et al. Probing the mechanical architecture of the vertebrate meiotic spindle. Nat Methods 6, 167–172 (2009). https://doi.org/10.1038/nmeth.1297
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1297
This article is cited by
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
-
Validation of the effects of TGF-β1 on tumor recurrence and prognosis through tumor retrieval and cell mechanical properties
Cancer Cell International (2014)
-
The F-actin and adherence-dependent mechanical differentiation of normal epithelial cells after TGF-β1-induced EMT (tEMT) using a microplate measurement system
Biomedical Microdevices (2014)
-
XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules
Nature Cell Biology (2013)
-
A single Drosophila embryo extract for the study of mitosis ex vivo
Nature Protocols (2013)