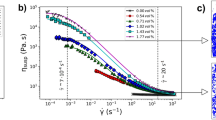
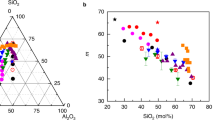
Abstract
Low-density zeolites collapse to the rigid amorphous state at temperatures that are well below the melting points of crystals of the same composition but of conventional density. Here we show, by using a range of experimental techniques, how the phenomenon of amorphization is time dependent, and how the dynamics of order–disorder transitions in zeolites under temperature and pressure are equivalent. As a result, thermobaric regions of instability can be charted, which are indicative of polyamorphism. Moreover, the boundaries of these zones depend on the rate at which temperature or pressure is ramped. By directly comparing the rheology of collapse with structural relaxation in equivalent melts, we conclude that zeolites amorphize like very strong liquids and, if compression occurs slowly, this is likely to lead to the synthesis of perfect glasses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sharma, S.M. & Sikka, S.K. Pressure-induced amorphisation of materials. Prog. Mater. Sci. 40, 1–77 (1996).
Richet, P. & Gillet, P. Pressure-induced amorphisation of minerals: a review. Eur. J. Mineral. 9, 907–933 (1997).
Cohen, M.H., Íñiguez, J. & Neaton, B. Flat bands and pressure amorphisation. J. Non-Cryst. Solids 307–310, 602–612 (2002).
Sciortino, F. et al. Crystal stability limits at positive and negative pressures, and crystal-to-glass transitions. Phys Rev. E 52, 6484–6491 (1995).
Ponyatovsky, E.G. & Barkolov, O.I. Pressure induced amorphous phases. Mater. Sci. Rep. 8, 147–191 (1992).
Mishma, O., Calvert, L.D. & Whalley E. 'Melting ice' I at 77 K and 10 kbar: a new method of making amorphous solids. Nature 310, 393–395 (1984).
Hemley, R.J., Jephcote, A.P., Mao, H.K., Ming, L.C. & Manghnani, M.H. Pressure-induced amorphisation of crystalline silica. Nature 334, 52–54 (1988).
Kingma, K.J., Hemley, R.J., Mao, H.-K. & Veblen, D.R. New high-pressure transformation in α-quartz. Phys. Rev. Lett. 70, 3927–3930 (1993).
Binggeli, N., Keskar, N.R. & Chelikowsky, J.R. Pressure-induced amorphisation, elastic instability and soft modes in alpha-quartz. Phys. Rev. B 49, 3075–3081 (1994).
Deb, S.K., Wilding, M., Somayazulu, M. & McMillan, P.F. Pressure-induced amorphisation and an amorphous-amorphous transition in densified porous silicon. Nature 414, 528–530 (2001).
Skinner, B.J. & Fahey, J.J. Observations on the inversion of stishotite to silica glass. J. Geophys. Res. 68, 5595–5604 (1963).
Richet, P. Superheating, melting & vitrification through decompression. Nature 331, 56–58 (1988).
Hemmati, M. et al. Crystalline-amorphous transition in silicate perovskites. Phys. Rev. B 51, 14841–14848 (1995).
Sankar, G. et al. Combined QuEXAFS-XRD: a new technique in high-temperature materials chemistry; an illustrative in situ study of zinc oxide enhanced solid state production of cordierite from a precurser zeolite. J. Phys. Chem. 97, 9550–9554 (1993).
Colyer, L.M. et al. In situ study of ceramic formation from Co2+ Cu2+ and Zn2+ exchanged zeolite-A using combined XRD/XAFS techniques. Nucl. Instr. Meth. Phys. Res. B 97, 107–110 (1995).
Colyer, L.M. Recrystallisation Processes in Transition Metal Exchanged Zeolite-A. Thesis, Univ. Keele (1996).
Colyer, L.M., Greaves, G.N., Carr, S.W. & Fox, K.K. Collapse and recrystallisation processes in zinc-exchanged zeolite-A: a combined x-ray diffraction, XAFS, and NMR study. J. Phys. Chem. 101, 10105–10114 (1997).
Greaves, G.N. in Phase Transitions and Self-Organisation in Electronic and Molecular Materials (eds Phillips, J.C. & Thorpe, M.F.) 225–246 (Kluwer/Plenum, New York, 2000).
Greaves, G.N. in Frontiers of High Pressure Research II: Application of High Pressure to Low Dimensional Electronic Materials (ed. Hochheimer, H.D.) 53–71 (Kluwer/Plenum, New York, 2001).
Dyer, A. An Introduction to Zeolite Molecular Sieves (Wiley, New York, 1988).
Dooryhee, E. et al. A study of cation environment and movement during dehydration and reduction of nickel-exchanged zeolite-Y by X-ray absorption and diffraction. J. Phys. Chem. 95, 1229–1236 (1991).
Hammonds, K.D., Deng, H., Heine, V. & Dove, M.T. How floppy modes give rise to adsorption sites in zeolites. Phys. Rev. Lett. 78, 3701–3704 (1997).
Dove, M.T., Hammonds, K.D. & Trachenko, K. in Rigidity Theory and Applications (eds Thorpe, M.F. & Duxbury, P.M.) 217–238 (Kluwer/Plenum, New York, 1999).
Villaescusa, L.A., Lightfoot, P., Teat, S.J. & Morris R.E. Variable-temperature microcrystal X-ray diffraction studies of negative thermal expansion in the pure silica zeolite IFR. J. Am. Chem. Soc. 123, 5453–5459 (2001).
Hazan, R.M. Zeolite molecular sieve 4A: anomalous compressibility and volume discontinuities at high pressure. Science 219, 1065–1067 (1983).
Toplis, M.J., Dingwell, D.B, Hess, K.-U. & Lenci, T. Viscosity, fragility, and configurational entropy of melts along the join SiO2-NaAlSiO4 . Am. Mineral. 82, 979–990 (1997).
Webb, S. & Dingwell, D.B. in Structure, Dynamics and Properties of Silicate Melts (Reviews in Mineralogy Vol. 32) (eds Stebbins, J.F., McMillan, P.F. & Dingwell, D.G.) 95–119 (Mineralogical Society of America, Washington DC, 1995).
Duran, J. Sands, Powders, and Grains 127–153 (Springer, New York, 1999).
Neuville, D.R. & Richet, P. Viscosity and mixing in molten (Ca, Mg) pyroxenes and garnets. Geochim. Cosmochim. Acta 55, 1011–1019 (1991).
Angell, C.A. in Relaxation in Complex Systems 3–11 (Office of Naval Research, Washington DC, 1985).
Lowenstein, W. The distribution of aluminium in the tetrahedra of silicates and aluminosilicates. Am. Mineral. 39, 92–96 (1954).
Kauzmann, W. The nature of the glassy state and the behaviour of liquids at low temperatures. Chem. Rev. 43, 219–256 (1948).
Acknowledgements
We thank C. A. Angell, M. H. Cohen, D. B. Dingwell, S. Webb, K. Walters and M. Wilding for discussions. The experimental work was financially supported from the UK Engineering and Physical Sciences Research Council and X-ray facilities at the Synchrotron Radiation Source, Daresbury Laboratory, UK. F.M. thanks the EU Framework 5 Programme for a studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Greaves, G., Meneau, F., Sapelkin, A. et al. The rheology of collapsing zeolites amorphized by temperature and pressure. Nature Mater 2, 622–629 (2003). https://doi.org/10.1038/nmat963
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat963
This article is cited by
-
Siliceous zeolite-derived topology of amorphous silica
Communications Chemistry (2023)
-
Thermal insulating performance and compressive strength of ZSM-5 zeolite ceramics by cold sintering process
Journal of Porous Materials (2023)
-
Accurate large-scale simulations of siliceous zeolites by neural network potentials
npj Computational Materials (2022)
-
Synthesis of Pure Silica Zeolites
Chemical Research in Chinese Universities (2022)
-
Structure and properties of densified silica glass: characterizing the order within disorder
NPG Asia Materials (2020)