Abstract
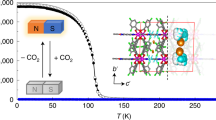
Interest in metal–organic open-framework structures has increased enormously in the past few years because of the potential benefits of using crystal engineering techniques to yield nanoporous materials with predictable structures and interesting properties. Here we report a new efficient methodology for the preparation of metal–organic open-framework magnetic structures based on the use of a persistent organic free radical (PTMTC), functionalized with three carboxylic groups. Using this approach, we create an open-framework structure Cu3(PTMTC)2(py)6(CH3CH2OH)2(H2O), which we call MOROF-1, combining very large pores (2.8–3.1 nm) with bulk magnetic ordering. MOROF-1 shows a reversible and highly selective solvent-induced 'shrinking–breathing' process involving large volume changes (25–35%) that strongly influence the magnetic properties of the material. This magnetic sponge-like behaviour could be the first stage of a new route towards magnetic solvent sensors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cheetham, A.K., Férey, G. & Loiseau, T. Open-framework inorganic materials. Angew. Chem. Int. Edn 38, 3269–3292 (1999).
Yaghi, O.M., Li, H., Davis, C., Richardson, D. & Groy, T.L. Synthetic strategies, structure patterns, and emerging properties in the chemistry of modular porous solids. Acc. Chem. Res. 31, 474–484 (1998).
Zaworotko, M. Nanoporous structures by design. Angew. Chem. Int. Edn 39, 3052–3054 (2000).
Noro, S.I., Kitagawa, S., Kondo, M. & Seki, K. A new, methane adsorbent, porous coordination polymer [{CuSiF6(4,4'-bipyridine)2}n].Angew. Chem. Int. Edn 12, 2081–2084 (2000).
Xu, X., Nieuwenhuyzen, M. & James, S.L. A nanoporous metal-organic framework based on bulky phosphane ligands. Angew. Chem. Int. Edn 41, 764–767 (2002).
Bennett, M.V., Beauvais, L.G., Shores, M.P. & Long, J.R. Expanded prussian blue analogues incorporating [Re6Se8(CN)6]3−/4− clusters: adjusting porosity via charge balance. J. Am. Chem. Soc. 123, 8022–8032 (2001).
Pschirer, N.G., Ciurtin, D.M., Smith, M.D., Bunz, U.H.F. & zur Loye, H.-C. Noninterpenetrating square-grid coordination polymers with dimensions of 25×25Å2 prepared by using N,N'-type ligands: the first chiral square grid coordination polymer. Angew. Chem. Int. Edn 41, 583–586 (2002).
Chen, B., Eddaoudi, M., Hyde, S.T., O'Keefe, M. & Yaghi, O.M. Interwoven metal-organic framework on a periodic minimal surface with extra-large pores. Science 291, 1021–1023 (2001).
Barthelet, K., Marrot, J., Riou, D. & Férey, G. A breathing hybrid organic-inorganic solid with very large pores and high magnetic characteristics. Angew. Chem. Int. Edn 41, 281–284 (2002).
Wynn, C.M., Albrecht, A.S., Landee, C.P., Turnbull, M.M. & Dodrill, B. Resonance in the nonlinear susceptibilities of Co3BTCA2(H2O)4, a molecular-based magnet. J. Solid State Chem. 159, 379–384 (2001).
Forster, P.M. & Cheetham, A.K. Open-framework nickel succinate, [Ni7(C4H4O4)6(OH)2(H2O)2]·2H2O: a new hybrid material with three dimensional Ni-O-Ni connectivity. Angew. Chem. Int. Edn 41, 457–459 (2002).
Cotton, F.A., Lin, C. & Murillo, C.A. Supramolecular arrays based on dimetal building units. Acc. Chem. Res. 34, 759–771 (2001).
Price, D.J., Tripp, S., Powell, A.K. & Wood, P.T. Hydrothermal synthesis, X-ray structure and complex magnetic behaviour of Ba4(C2O4)Cl2[{Fe(C2O4)(OH)}4]. Chem. Eur. J. 7, 200–208 (2001).
Chui, S.S.-Y., Lo, S.M.-F., Charmant, J.P.H., Orpen, A.G. & Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n . Science 283, 1148–1150 (1999).
Moulton, B., Lu, J., Hajndl, R., Hariharan, S & Zaworotko, M.J. Crystal Engineering of a nanoscale Kagomé lattice. Angew. Chem. Int. Edn 41, 2821–2824 (2002).
Kim, J. et al. Assembly of metal-organic frameworks from large organic and inorganic secondary building units: new examples and simplifying principles for complex structures. J. Am. Chem. Soc. 123, 8329–8247 (2001).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Beauvais, L.G. & Long, J.R. Co3[Co(CN)5]2: A microporous magnet with ordering temperature of 38 K. J. Am. Chem. Soc. 124, 12096–12097 (2002).
Laget, V., Hornick, C., Rabu, P., Drillon, M. & Ziessel, R. Molecular magnets hybrid organic-inorganic layered compounds with very long-range ferromagnetism. Coordin. Chem. Rev. 178, 1533–1553 (1998).
Ballester, M. Inert free radicals: a unique trivalent carbon species. Acc. Chem. Res. 12, 380 (1985).
Pech, R. & Pickardt, J. Catena-triaqua-μ-[1,3,5-benzenetricarboxylato(2-)]-copper(II). Acta Cryst. C 44, 992–994 (1988).
Abourahma, H., Moulton, B., Kravtsov, V. & Zaworotko, M.J. Supramolecular isomerism in coordination compounds: nanoscale molecular hexagons and chains. J. Am. Chem. Soc. 124, 9990–9991 (2002).
Min, K.S. & Suh, M.P. Self-assembly and selective guest binding of three-dimensional open-framework solids form a macrocyclic complex as a trifunctional metal building block. Chem. Eur. J. 7, 303–313 (2001).
Lu, J.Y. & Babb, A.M. An extremely stable open-framework metal-organic polymer with expandable structure and selective adsorption capability. Chem. Commun. 1340–1341 (2002).
Kitagawa, S. et al. Novel flexible frameworks of porous (II) coordination polymers that show selective guest adsorption based on the switching of hydrogen-bond pairs of amide groups. Chem. Eur. J. 8, 3587–3600 (2002).
Biradha, K. & Fujita, M. A springlike 3D-coordination network that shrinks or swells in a crystal-to-crystal manner upon guest removal or re-adsorption. Angew. Chem. Int. Edn 41, 3392–3395 (2002).
Li, H., Davis, C.E., Groy, T.L., Kelley, D.G. & Yaghi, O.M. Coordinatively unsaturated metal centers in the extended porous framework of Zn3(BDC)3·6H2O (BDC=1,4-benzene dicarboxylate). J. Am. Chem. Soc. 120, 2186–2187 (1998).
Maspoch, D., Ruiz-Molina, D., Wurst, K., Rovira, C. & Veciana, J. A very bulky carboxylic perchlorotriphenylmethyl radical as a novel ligand for transition metal complexes. A new spin frustrated metal system. Chem. Commun. 2958–2959 (2002).
Larionova, J., Mombelli, B., Sanchiz, J. & Kahn, O. Magnetic properties of the two-dimensional bimetallic compounds (NBu4)[MIIRuIII(ox)3] (NBu4=Tetra-n-butylammonium; M=Mn, Fe, Cu; ox=oxalate). Inorg. Chem. 37, 679–684 (1998).
Kahn, O. in Molecular Magnetism (ed. Kahn, O.) 251–332 (VCH, New York, 1993).
Caneschi, A., Gatteschi, D., Lalioti, N., Sangregorio, C. & Sessoli, R. Supramolecular interactions and magnetism of metal-radical chains. J. Chem. Soc. Dalton Trans. 2000, 3907–3912 (2000).
Ivamura, H. & Inoue, K. in Magnetism: Molecules to Materials Vol. III (eds Miller, J.S. & Drillon, M.) 61–108 (Wiley-VCH, Weinheim, 2001).
Acknowledgements
This work was supported by the Programa Nacional de Materiales of the Dirección General de Investigación (Spain), under project MAGMOL. D.M. is grateful to the Generalitat de Catalunya for a predoctoral grant. We thank P. Gerbier of the Université Montpellier for TG-MS experiments and X. Alcobé of the Universitat de Barcelona for X-ray powder diffraction measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Figure 1S Magnetization value as a function of the applied field at a temperature of 2.5 K for MOROF-1
Figure 2S Reversible magnetic behavior of the amorphous and evacuated phase in contact with ethanol liquid, plot of magnetization value as a function of the applied field at a temperature of 2.5 K (PDF 891 kb)
Figure 3S Plots of χ'MT (top) and χ"MT (bottom) as a function of the temperature for MOROF-1 at the indicated frequencies
Figure 4S χT value as a function of the temperature for MOROF-1
Figure 5S (Left) χT value as a function of the temperature for complex Cu(PTMMC)2(H2O)3.
Figure 6S Powder X-ray diffractogram of MOROF-1 (red) and MOOF-1 (black).
Structure The crystal structure of MOROF-1.
Rights and permissions
About this article
Cite this article
Maspoch, D., Ruiz-Molina, D., Wurst, K. et al. A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties. Nature Mater 2, 190–195 (2003). https://doi.org/10.1038/nmat834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat834
This article is cited by
-
Recent progress of metal–organic frameworks as sensors in (bio)analytical fields: towards real-world applications
Analytical and Bioanalytical Chemistry (2023)
-
Roadmap of amorphous metal-organic framework for electrochemical energy conversion and storage
Nano Research (2023)
-
Giant single-crystal-to-single-crystal transformations associated with chiral interconversion induced by elimination of chelating ligands
Nature Communications (2021)
-
Controlling pairing of π-conjugated electrons in 2D covalent organic radical frameworks via in-plane strain
Nature Communications (2021)
-
A chromium(III) bis-acetylide complex containing a trans-diethyl-ethylenedithio-substituted tetrathiafulvalene (TTF) derivative: synthesis, crystal structures, and magnetic properties
Transition Metal Chemistry (2021)