Abstract
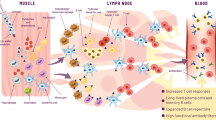
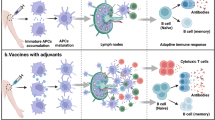
A major challenge in vaccine formulations is the stimulation of both the humoral and cellular immune response for well-defined antigens with high efficacy and safety. Adjuvant research has focused on developing particulate carriers to model the sizes, shapes and compositions of microbes or diseased cells, but not antigen fluidity and pliability. Here, we develop Pickering emulsions—that is, particle-stabilized emulsions that retain the force-dependent deformability and lateral mobility of presented antigens while displaying high biosafety and antigen-loading capabilities. Compared with solid particles and conventional surfactant-stabilized emulsions, the optimized Pickering emulsions enhance the recruitment, antigen uptake and activation of antigen-presenting cells, potently stimulating both humoral and cellular adaptive responses, and thus increasing the survival of mice upon lethal challenge. The pliability and lateral mobility of antigen-loaded Pickering emulsions may provide a facile, effective, safe and broadly applicable strategy to enhance adaptive immunity against infections and diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat. Med. 19, 1597–1608 (2013).
Gautam, R. et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533, 105–109 (2016).
Irvine, D. J., Swartz, M. A. & Szeto, G. L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 12, 978–990 (2013).
St John, A. L. et al. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 11, 250–257 (2012).
Lynn, G. M. et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotech. 33, 1201–1210 (2015).
Alon, R. & Dustin, M. L. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity 26, 17–27 (2007).
Deeg, J. et al. T cell activation is determined by the number of presented antigens. Nano Lett. 13, 5619–5626 (2013).
Platzman, I., Janiesch, J. W. & Spatz, J. P. Synthesis of nanostructured and biofunctionalized water-in-oil droplets as tools for homing T cells. J. Am. Chem. Soc. 135, 3339–3342 (2013).
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 (1998).
Blachère, N. E., Darnell, R. B. & Albert, M. L. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 3, e185 (2005).
O’Connor, R. S. et al. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 189, 1330–1339 (2012).
Ben M’Barek, K. et al. Phagocytosis of immunoglobulin-coated emulsion droplets. Biomaterials 51, 270–277 (2015).
Frelichowska, J. et al. Pickering w/o emulsions: drug release and topical delivery. Int. J. Pharm. 368, 7–15 (2009).
Rodriguez-Arco, L., Li, M. & Mann, S. Phagocytosis-inspired behaviour in synthetic protocell communities of compartmentalized colloidal objects. Nat. Mater. 16, 857–863 (2017).
Dewey, D. C. et al. Bioreactor droplets from liposome-stabilized all-aqueous emulsions. Nat. Commun. 5, 4670 (2014).
Lin, Y. et al. Nanoparticle assembly and transport at liquid-liquid interfaces. Science 299, 226–229 (2003).
Brito, L. A., Malyala, P. & O’Hagan, D. T. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin. Immunol. 25, 130–145 (2013).
Lalanne, M. et al. Metabolism evaluation of biomimetic prodrugs by in vitro models and mass spectrometry. Int. J. Pharm. 379, 235–243 (2009).
Christenson, H. K. Capillary condensation in systems of immiscible liquids. J. Colloid Interface Sci. 104, 234–249 (1985).
Niikura, K. et al. Gold nanoparticles coated with semi-fluorinated oligo(ethylene glycol) produce sub-100 nm nanoparticle vesicles without templates. J. Am. Chem. Soc. 134, 7632–7635 (2012).
Ghosh, S. & Coupland, J. N. Factors affecting the freeze–thaw stability of emulsions. Food Hydrocolloids 22, 105–111 (2008).
Nejadnik, M. R. et al. Adsorption of pluronic F-127 on surfaces with different hydrophobicities probed by quartz crystal microbalance with dissipation. Langmuir 25, 6245–6249 (2009).
Johannsmann, D., Reviakine, I. & Richter, R. P. Dissipation in films of adsorbed nanospheres studied by quartz crystal microbalance (QCM). Anal. Chem. 81, 8167–8176 (2009).
Rimaniol, A. C., Gras, G. & Clayette, P. In vitro interactions between macrophages and aluminum-containing adjuvants. Vaccine 25, 6784–6792 (2007).
Vasir, J. K. & Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 59, 718–728 (2007).
Posey, A. D. Jr et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016).
Zhang, H. et al. MUC1 and survivin combination tumor gene vaccine generates specific immune responses and anti-tumor effects in a murine melanoma model. Vaccine 34, 2648–2655 (2016).
Teramoto, K. et al. Predictive biomarkers and effectiveness of MUC1-targeted dendritic-cell-based vaccine in patients with refractory non-small cell lung cancer. Ther. Adv. Med. Oncol. 9, 147–157 (2017).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Luo, N. et al. PEGylated graphene oxide elicits strong immunological responses despite surface passivation. Nat. Commun. 8, 14537 (2017).
Liu, J., Wickramaratne, N. P., Qiao, S. Z. & Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 14, 763–774 (2015).
Bencherif, S. A. et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 6, 7556 (2015).
Sheridan, C. Exosome cancer diagnostic reaches market. Nat. Biotech. 34, 359–360 (2016).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Schubert, S., Delaney, J. J. T. & Schubert, U. S. Nanoprecipitation and nanoformulation of polymers: from history to powerful possibilities beyond poly(lactic acid). Soft Matter 7, 1581–1588 (2011).
Calabro, S. et al. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 31, 3363–3369 (2013).
Galli, G. et al. Adjuvanted H5N1 vaccine induces early CD4 + T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl Acad. Sci. USA 106, 3877–3882 (2009).
Zhang, W. et al. Immune responses to vaccines involving a combined antigen–nanoparticle mixture and nanoparticle-encapsulated antigen formulation. Biomaterials 35, 6086–6097 (2014).
Zolnik, B. S. & Burgess, D. J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 122, 338–344 (2007).
Sarti, F. et al. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials 32, 4052–4057 (2011).
Torres, M. P. et al. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater. 7, 2857–2864 (2011).
Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646 (2013).
Morel, S. et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29, 2461–2473 (2011).
Calabro, S. et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823 (2011).
Deenick, E. K. et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253 (2010).
Sage, P. T., Francisco, L. M., Carman, C. V. & Sharpe, A. H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2013).
Acknowledgements
We thank T. Ngai from Department of Chemistry, Chinese University of Hong Kong, for his unyielding support in the preparation and stability evaluations of Pickering emulsion. We express our appreciation of S. Li and J. Zhang from Center for Biological Imaging (CBI), Institute of Biophysics, Chinese Academy of Science for their help in taking and analysing SIM and Cyro-SEM images. Our gratitude also goes to M. Wang and P. Jiao, from Center of Biomedical Analysis, Tsinghua University, for their support in the operation and analysis of flow cytometer in this manuscript. We also want to thank X. Yu from National Engineering Laboratory for AIDS Vaccine, College of Life Science, Jilin University, for B16/MUC1 tumour cells. This work was supported by the National Science and Technology Major Project (No. 2014ZX09102045) and National Natural Science Foundation of China project (21576268).
Author information
Authors and Affiliations
Contributions
J.W. and Y.X. conceived and designed the experiments. Y.X. and Y.D. carried out the experiments. G.M., X.C., J.W. and W.W. contributed ideas and suggestions for optimization and characterization of Pickering emulsion and in vivo evaluation and data analysis. T.W. and Y.X. conducted the comparison of PPAS and APDC. X.M., W.A. and A.G. facilitated evaluation of the influenza challenge model. S.L. assisted in taking and analysing microscope images. Y.X. wrote the manuscript. H.Y. facilitated the data and file processing. All authors discussed the results and commented on the manuscript. G.M., Z.S., W.W. and J.W. revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 62030 kb)
Supplementary Information
Supplementary movie 1 (MP4 48802 kb)
Supplementary Information
Supplementary movie 2 (MP4 6226 kb)
Supplementary Information
Supplementary movie 3 (MP4 8957 kb)
Supplementary Information
Supplementary movie 4 (MP4 11257 kb)
Supplementary Information
Supplementary movie 5 (MP4 3540 kb)
Rights and permissions
About this article
Cite this article
Xia, Y., Wu, J., Wei, W. et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 17, 187–194 (2018). https://doi.org/10.1038/nmat5057
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat5057
This article is cited by
-
Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies
npj Vaccines (2024)
-
Nanovaccine-based strategies for lymph node targeted delivery and imaging in tumor immunotherapy
Journal of Nanobiotechnology (2023)
-
Engineering nanomaterial physical characteristics for cancer immunotherapy
Nature Reviews Bioengineering (2023)
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)
-
A Comprehensive Investigation on Ho Wood Essential Oil Solution or Gel Using Pickering Systems
Journal of Polymers and the Environment (2023)