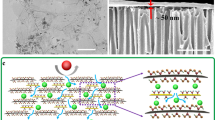
Abstract
Graphene oxide (GO) membranes continue to attract intense interest due to their unique molecular sieving properties combined with fast permeation1,2,3,4,5,6,7,8,9. However, their use is limited to aqueous solutions because GO membranes appear impermeable to organic solvents1, a phenomenon not yet fully understood. Here, we report efficient and fast filtration of organic solutions through GO laminates containing smooth two-dimensional (2D) capillaries made from large (10–20 μm) flakes. Without modification of sieving characteristics, these membranes can be made exceptionally thin, down to ∼10 nm, which translates into fast water and organic solvent permeation. We attribute organic solvent permeation and sieving properties to randomly distributed pinholes interconnected by short graphene channels with a width of 1 nm. With increasing membrane thickness, organic solvent permeation rates decay exponentially but water continues to permeate quickly, in agreement with previous reports1,2,3,4. The potential of ultrathin GO laminates for organic solvent nanofiltration is demonstrated by showing >99.9% rejection of small molecular weight organic dyes dissolved in methanol. Our work significantly expands possibilities for the use of GO membranes in purification and filtration technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335, 442–444 (2012).
Sun, P., Wang, K. & Zhu, H. Recent developments in graphene-based membranes: structure, mass-transport mechanism and potential applications. Adv. Mater. 28, 2287–2310 (2016).
Liu, G., Jin, W. & Xu, N. Graphene-based membranes. Chem. Soc. Rev. 44, 5016–5030 (2015).
Fathizadeh, M., Xu, W. L., Zhou, F., Yoon, Y. & Yu, M. Graphene oxide: a novel 2-dimensional material in membrane separation for water purification. Adv. Mater. Interfaces 4, 1600918 (2017).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Li, H. et al. Ultrathin, molecular-sieving graphene oxide membranes for selective hydrogen separation. Science 342, 95–98 (2013).
Akbari, A. et al. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat. Commun. 7, 10891 (2016).
Abraham, J. et al. Tuneable sieving of ions using graphene oxide membranes. Nat. Nanotech. 12, 546–550 (2017).
Hong, S. et al. Scalable graphene-based membranes for ionic sieving with ultrahigh charge selectivity. Nano Lett. 17, 728–732 (2017).
Mulder, J. Basic Principles of Membrane Technology (Springer Science & Business Media, 2012).
Koros, W. J. & Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 16, 289–297 (2017).
Wang, L. et al. Molecular valves for controlling gas phase transport made from discrete ångström-sized pores in graphene. Nat. Nanotech. 10, 785–790 (2015).
Jain, T. et al. Heterogeneous sub-continuum ionic transport in statistically isolated graphene nanopores. Nat. Nanotech. 10, 1053–1057 (2015).
Celebi, K. et al. Ultimate permeation across atomically thin porous graphene. Science 344, 289–292 (2014).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013).
Marchetti, P., Jimenez Solomon, M. F., Szekely, G. & Livingston, A. G. Molecular separation with organic solvent nanofiltration: a critical review. Chem. Rev. 114, 10735–10806 (2014).
Vandezande, P., Gevers, L. E. & Vankelecom, I. F. Solvent resistant nanofiltration: separating on a molecular level. Chem. Soc. Rev. 37, 365–405 (2008).
Jimenez-Solomon, M. F., Song, Q., Jelfs, K. E., Munoz-Ibanez, M. & Livingston, A. G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 15, 760–767 (2016).
Karan, S., Jiang, Z. & Livingston, A. G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 348, 1347–1351 (2015).
Huang, K. et al. A graphene oxide membrane with highly selective molecular separation of aqueous organic solution. Angew. Chem. Int. Ed. 53, 6929–6932 (2014).
Huang, L., Li, Y., Zhou, Q., Yuan, W. & Shi, G. Graphene oxide membranes with tunable semipermeability in organic solvents. Adv. Mater. 27, 3797–3802 (2015).
Aba, N. F. D., Chong, J. Y., Wang, B., Mattevi, C. & Li, K. Graphene oxide membranes on ceramic hollow fibers—microstructural stability and nanofiltration performance. J. Membr. Sci. 484, 87–94 (2015).
Huang, L. et al. Reduced graphene oxide membranes for ultrafast organic solvent nanofiltration. Adv. Mater. 28, 8669–8674 (2016).
Lin, X. et al. Fabrication of highly-aligned, conductive, and strong graphene papers using ultralarge graphene oxide sheets. ACS Nano 6, 10708–10719 (2012).
Wu, H., Gong, Q., Olson, D. H. & Li, J. Commensurate adsorption of hydrocarbons and alcohols in microporous metal organic frameworks. Chem. Rev. 112, 836–868 (2012).
Secchi, E. et al. Massive radius-dependent flow slippage in carbon nanotubes. Nature 537, 210–213 (2016).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Wilson, N. R. et al. Graphene oxide: structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 3, 2547–2556 (2009).
Loh, K. P., Bao, Q., Eda, G. & Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2, 1015–1024 (2010).
Dai, H., Liu, S., Zhao, M., Xu, Z. & Yang, X. Interfacial friction of ethanol–water mixtures in graphene pores. Microfluid. Nanofluid. 20, 141 (2016).
Acknowledgements
This work was supported by the Royal Society, Engineering and Physical Sciences Research Council, UK (EP/K016946/1), Lloyd’s Register Foundation, and European Research Council (contract 679689). Q.Y. acknowledges support from the China Scholarship Council. We thank P. Bentley for assisting with XPS measurements, J. Waters for X-ray measurements, and K. Huang for assisting in setting up the cold trap for filtration experiments.
Author information
Authors and Affiliations
Contributions
R.R.N. and Y.S. designed and supervised the project. Q.Y., Y.S. and C.C prepared the samples, performed the measurements and carried out the analysis with help from R.R.N. C.T.C. and K.H. helped in sample preparation, characterization and data analysis. J.C.Z. and A.P. contributed to XPS characterization. V.G.K. and A.N.G. contributed to optical measurements. F.G., F.C.W. and A.K.G. contributed to theoretical modelling. Y.S., A.N.G., A.K.G. and R.R.N. wrote the manuscript. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1012 kb)
Supplementary Information
Supplementary movie 1 (MP4 19635 kb)
Rights and permissions
About this article
Cite this article
Yang, Q., Su, Y., Chi, C. et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nature Mater 16, 1198–1202 (2017). https://doi.org/10.1038/nmat5025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat5025
This article is cited by
-
Unlocking osmotic energy harvesting potential in challenging real-world hypersaline environments through vermiculite-based hetero-nanochannels
Nature Communications (2024)
-
Graphene oxide-based membranes for water desalination and purification
npj 2D Materials and Applications (2024)
-
Ultrathin organosiloxane membrane for precision organic solvent nanofiltration
Nature Communications (2024)
-
Cyclodextrin nanofilms with hydrophobic and hydrophilic channels for solvent permeation and molecular sieving
Nano Research (2024)
-
Internally functionalized MnO2 nanotubes in modification of thin-film nanocomposite membranes for water and wastewater treatment
Iranian Polymer Journal (2024)