Abstract
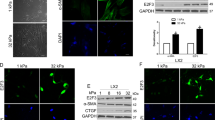
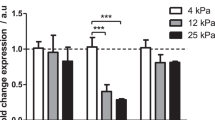
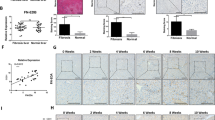
The role of pathological angiogenesis on liver fibrogenesis is still unknown. Here, we developed fibrotic microniches (FμNs) that recapitulate the interaction of liver sinusoid endothelial cells (LSECs) and hepatic stellate cells (HSCs). We investigated how the mechanical properties of their substrates affect the formation of capillary-like structures and how they relate to the progression of angiogenesis during liver fibrosis. Differences in cell response in the FμNs were synonymous of the early and late stages of liver fibrosis. The stiffness of the early-stage FμNs was significantly elevated due to condensation of collagen fibrils induced by angiogenesis, and led to activation of HSCs by LSECs. We utilized these FμNs to understand the response to anti-angiogenic drugs, and it was evident that these drugs were effective only for early-stage liver fibrosis in vitro and in an in vivo mouse model of liver fibrosis. Late-stage liver fibrosis was not reversed following treatment with anti-angiogenic drugs but rather with inhibitors of collagen condensation. Our work reveals stage-specific angiogenesis-induced liver fibrogenesis via a previously unrevealed mechanotransduction mechanism which may offer precise intervention strategies targeting stage-specific disease progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Friedman, S. L., Sheppard, D., Duffield, J. S. & Violette, S. Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167sr161 (2013).
DiPietro, L. A. Angiogenesis and scar formation in healing wounds. Curr. Opin. Rheumatol. 25, 87–91 (2013).
Hanumegowda, C., Farkas, L. & Kolb, M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest 142, 200–207 (2012).
Ehling, J. et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut 63, 1960–1971 (2014).
Thabut, D. & Shah, V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J. Hepatol. 53, 976–980 (2010).
Tugues, S. et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology 46, 1919–1926 (2007).
Mejias, M. et al. Beneficial effects of Sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49, 1245–1256 (2009).
Karimian, G. et al. Attenuation of hepatic fibrosis through captopril and enalapril in the livers of bile duct ligated rats. Biomed. Pharmacother. 62, 312–316 (2008).
Patsenker, E. et al. Pharmacological inhibition of integrin αvβ3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology 50, 1501–1511 (2009).
Yang, L. et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology 146, 1339–1350.e1 (2014).
Kantari-Mimoun, C. et al. Resolution of liver fibrosis requires myeloid cell-driven sinusoidal angiogenesis. Hepatology 61, 2042–2055 (2015).
Garcia-Tsao, G., Friedman, S., Iredale, J. & Pinzani, M. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology 51, 1445–1449 (2010).
Schaffner, F. & Poper, H. Capillarization of hepatic sinusoids in man. Gastroenterology 44, 239–242 (1963).
Xie, G. et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 142, 918–927.e6 (2012).
DeLeve, L. D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 61, 1740–1746 (2015).
Rockey, D. C. Vascular mediators in the injured liver. Hepatology 37, 4–12 (2003).
Duscher, D. et al. Mechanotransduction and fibrosis. J. Biomech. 47, 1997–2005 (2014).
Kilarski, W. W. et al. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med. 15, 657–664 (2009).
Korff, T. & Augustin, H. G. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J. Cell Sci. 112, 3249–3258 (1999).
Bishop, P. N. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp. Eye Res. 133, 30–36 (2015).
Kolodney, M. S. & Wysolmerski, R. B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 117, 73–82 (1992).
Prasain, N. & Stevens, T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc. Res. 77, 53–63 (2009).
Melgar-Lesmes, P. et al. Vascular endothelial growth factor and angiopoietin-2 play a major role in the pathogenesis of vascular leakage in cirrhotic rats. Gut 58, 285–292 (2009).
Huynh, J. et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 3, 112ra122 (2011).
Tornavaca, O. et al. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J. Cell Biol. 208, 821–838 (2015).
Zhao, X. & Guan, J.-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 63, 610–615 (2011).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Narine, K. et al. Growth factor modulation of fibroblast proliferation, differentiation, and invasion: implications for tissue valve engineering. Tissue Eng. 12, 2707–2716 (2006).
Weng, S., Shao, Y., Chen, W. & Fu, J. Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis. Nat. Mater. 15, 961–967 (2016).
Wang, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005).
Ouyang, M., Sun, J., Chien, S. & Wang, Y. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc. Natl Acad. Sci. USA 105, 14353–14358 (2008).
Ikeda, K. et al. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J. Biol. Chem. 277, 19206–19212 (2002).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Olaso, E. et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J. Clin. Invest. 108, 1369–1378 (2001).
Leitinger, B. Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 310, 39–87 (2014).
Payne, L. S. & Huang, P. H. Discoidin domain receptor 2 signaling networks and therapy in lung cancer. J. Thorac. Oncol. 9, 900–904 (2014).
Abdalla, M., Goc, A., Segar, L. & Somanath, P. R. Akt1 mediates α-smooth muscle actin expression and myofibroblast differentiation via myocardin and serum response factor. J. Biol. Chem. 288, 33483–33493 (2013).
Sohrabpour, A. A., Mohamadnejad, M. & Malekzadeh, R. Review article: the reversibility of cirrhosis. Aliment. Pharmacol. Ther. 36, 824–832 (2012).
Barry-Hamilton, V. et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 (2010).
Chaudhuri, O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 13, 970–978 (2014).
Engler, A. et al. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 86, 617–628 (2004).
Simonetto, D. A. et al. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology 61, 648–659 (2015).
Mederacke, I. et al. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat. Protoc. 10, 305–315 (2015).
Yang, Y., Wang, K., Gu, X. & Leong, K. W. Biophysical regulation of cell behavior—cross talk between substrate stiffness and nanotopography. Engineering 3, 36–54 (2017).
Wang, B. et al. Substrate stiffness orchestrates epithelial cellular heterogeneity with controlled proliferative pattern via E-cadherin/β-catenin mechanotransduction. Acta Biomater. 41, 169–180 (2016).
Zhao, H. et al. Microengineered in vitro model of cardiac fibrosis through modulating myofibroblast mechanotransduction. Biofabrication 6, 045009 (2014).
Kızılel, S., Sawardecker, E., Teymour, F. & Pérez-Luna, V. H. Sequential formation of covalently bonded hydrogel multilayers through surface initiated photopolymerization. Biomaterials 27, 1209–1215 (2006).
Gutekunst, S. B. et al. Influence of the PDMS substrate stiffness on the adhesion of Acanthamoeba castellanii. Beilstein J. Nanotech. 5, 1393–1398 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Wang, L. et al. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010).
Kee, K. et al. Human DAZL, DAZ and BOULE genes modulate primordial germ cell and haploid gamete formation. Nature 462, 222–225 (2009).
Zeng, Y. et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 59, 53–65 (2015).
Speier, S. et al. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat. Protoc. 3, 1278–1286 (2008).
Zhao, H. et al. Bi-content micro-collagen chip provides contractility-based biomechanical readout for phenotypic drug screening with expanded and profiled targets. Lab Chip 15, 3481–3494 (2015).
Acknowledgements
We would like to acknowledge the sequencing core facility, NIKON Biological Imaging Center, Animal Core Facility and Center of Biomedical Analysis at Tsinghua University for technical assistance. This work is financially supported by the National Natural Science Foundation of China (81522022) and National Key R&D Program of China (2017YFA0104901).
Author information
Authors and Affiliations
Contributions
L.L., Z.Y. and Y.D. conceived and designed the experiments; L.L. conducted the in vitro FμN experiments with help from H.Z., L.Zhu and B.W.; H.Y., B.W. and L.Zhou helped to establish the hydrogel system and performed the AFM experiments, while Y.S. and T.X. provided technical support; D.L. helped in flow analysis; Z.Y. and L.L. designed and performed the mouse experiments and data processing; Bioinformatics analysis was performed by L.L. and Y.X.; W.H. and C.H. provided clinical consultation; L.L., Z.Y. and Y.D. wrote the manuscript, which W.H., C.H., H.Y., X.Y. helped to revise; Y.D. is the principal investigator of the supporting grants.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2793 kb)
Supplementary Information
Supplementary movie 1 (MP4 48722 kb)
Supplementary Information
Supplementary movie 2 (MP4 4434 kb)
Supplementary Information
Supplementary movie 3 (MP4 5042 kb)
Supplementary Information
Supplementary movie 4 (MP4 16961 kb)
Supplementary Information
Supplementary movie 5 (MP4 12312 kb)
Supplementary Information
Supplementary movie 6 (MP4 8414 kb)
Supplementary Information
Supplementary movie 7 (MP4 2734 kb)
Supplementary Information
Supplementary table 1 (XLSX 3355 kb)
Supplementary Information
Supplementary table 2 (XLSX 64 kb)
Supplementary Information
Supplementary table 3 (XLSX 43 kb)
Rights and permissions
About this article
Cite this article
Liu, L., You, Z., Yu, H. et al. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nature Mater 16, 1252–1261 (2017). https://doi.org/10.1038/nmat5024
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat5024
This article is cited by
-
Biophysics in tumor growth and progression: from single mechano-sensitive molecules to mechanomedicine
Oncogene (2023)
-
Expansion of human megakaryocyte-biased hematopoietic stem cells by biomimetic Microniche
Nature Communications (2023)
-
Downregulation of miR-20b-5p Contributes to the Progression of Liver Fibrosis via the STAT3 Signaling Pathway In Vivo and In Vitro
Digestive Diseases and Sciences (2023)
-
Allometrically scaling tissue forces drive pathological foreign-body responses to implants via Rac2-activated myeloid cells
Nature Biomedical Engineering (2023)
-
LncRNA Airn maintains LSEC differentiation to alleviate liver fibrosis via the KLF2-eNOS-sGC pathway
BMC Medicine (2022)