Abstract
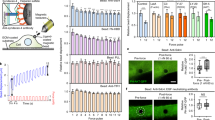
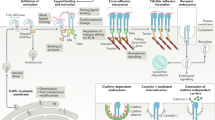
Epidermal growth factor receptor (EGFR) interacts with integrins during cell spreading and motility, but little is known about the role of EGFR in these mechanosensing processes. Here we show, using two different cell lines, that in serum- and EGF-free conditions, EGFR or HER2 activity increase spreading and rigidity-sensing contractions on rigid, but not soft, substrates. Contractions peak after 15–20 min, but diminish by tenfold after 4 h. Addition of EGF at that point increases spreading and contractions, but this can be blocked by myosin-II inhibition. We further show that EGFR and HER2 are activated through phosphorylation by Src family kinases (SFK). On soft surfaces, neither EGFR inhibition nor EGF stimulation have any effect on cell motility. Thus, EGFR or HER2 can catalyse rigidity sensing after associating with nascent adhesions under rigidity-dependent tension downstream of SFK activity. This has broad implications for the roles of EGFR and HER2 in the absence of EGF both for normal and cancerous growth.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wang, H. B., Dembo, M. & Wang, Y. L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 279, C1345–C1350 (2000).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Lo, C. M., Wang, H. B., Dembo, M. & Wang, Y. L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 (2000).
Elosegui-Artola, A. et al. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 13, 631–637 (2014).
Ghassemi, S. et al. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc. Natl Acad. Sci. USA 109, 5328–5333 (2012).
Wolfenson, H. et al. Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat. Cell Biol. 18, 33–42 (2016).
Meacci, G. et al. α-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol. Biol. Cell 27, 3471–3479 (2016).
Yang, B. et al. Mechanosensing controlled directly by tyrosine kinases. Nano Lett. 16, 5951–5961 (2016).
Sato, K.-I. Cellular functions regulated by phosphorylation of EGFR on Tyr845. Int. J. Mol. Sci. 14, 10761–10790 (2013).
Bill, H. M. et al. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol. Cell. Biol. 24, 8586–8599 (2004).
Huveneers, S. & Danen, E. H. J. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069 (2009).
Eberwein, P. et al. Modulation of focal adhesion constituents and their down-stream events by EGF: on the cross-talk of integrins and growth factor receptors. Biochim. Biophys. Acta 1853, 2183–2198 (2015).
Schneider, I. C., Hays, C. K. & Waterman, C. M. Epidermal growth factor-induced contraction regulates paxillin phosphorylation to temporally separate traction generation from de-adhesion. Mol. Biol. Cell 20, 3155–3167 (2009).
Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26, 6469–6487 (2007).
Iwabu, A., Smith, K., Allen, F. D., Lauffenburger, D. A. & Wells, A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J. Biol. Chem. 279, 14551–14560 (2004).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2, 793–805 (2001).
Wolfenson, H., Lavelin, I. & Geiger, B. Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell 24, 447–458 (2013).
Ménard, S., Tagliabue, E., Campiglio, M. & Pupa, S. M. Role of HER2 gene overexpression in breast carcinoma. J. Cell. Physiol. 182, 150–162 (2000).
Giannone, G. et al. Periodic lamellipodial contractions correlate with rearward actin waves. Cell 116, 431–443 (2004).
Cabodi, S. et al. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem. Soc. Trans. 32, 438–442 (2004).
Yamada, K. M. & Even-Ram, S. Integrin regulation of growth factor receptors. Nat. Cell Biol. 4, E75–E76 (2002).
Balanis, N. & Carlin, C. R. Mutual cross-talk between fibronectin integrins and the EGF receptor: molecular basis and biological significance. Cell. Logist. 2, 46–51 (2012).
Cai, Y. et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 91, 3907–3920 (2006).
Seshacharyulu, P. et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 16, 15–31 (2012).
Xu, K.-P., Yin, J. & Yu, F.-S. X. SRC-family tyrosine kinases in wound- and ligand-induced epidermal growth factor receptor activation in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 47, 2832–2839 (2006).
Iskratsch, T. et al. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev. Cell 27, 545–559 (2013).
Lauffenburger, D. A. & Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Adelstein, R. S. & Anne Conti, M. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature 256, 597–598 (1975).
Matsui, T. et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 15, 2208–2216 (1996).
Kim, J.-H. & Asthagiri, A. R. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J. Cell Sci. 124, 1280–1287 (2011).
Stuurman, N., Edelstein, A. D., Amodaj, N., Hoover, K. H. & Vale, R. D. Computer Control of Microscopes using μManager. Curr. Protoc. Mol. Biol. Ed. Unit14.20 (2010).
Schoen, I., Hu, W., Klotzsch, E. & Vogel, V. Probing cellular traction forces by micropillar arrays: contribution of substrate warping to pillar deflection. Nano Lett. 10, 1823–1830 (2010).
Ghibaudo, M. et al. Traction forces and rigidity sensing regulate cell functions. Soft Matter 4, 1836–1843 (2008).
Palchesko, R. N., Zhang, L., Sun, Y. & Feinberg, A. W. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 7, e51499 (2012).
Su, J., Muranjan, M. & Sap, J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9, 505–511 (1999).
Acknowledgements
We thank all the members of the Sheetz lab for their help. Especially M. X. Yao for preparing EGFR and HER2 mutants supported by MBI. This work was funded by National Institutes of Health (NIH) grant ‘Analysis of 120 nm local contractions linked to rigidity sensing’ (1 R01 GM100282-01), National Institutes of Health (NIH) grant ‘Tropomyosin and tyrosine kinases in mechanics of cancer’ (5 R01 GM113022-02) and by the NIH Common Fund Nanomedicine programme (PN2 EY016586). H.W. was supported by a Marie Curie International Outgoing Fellowship within the Seventh European Commission Framework Programme (PIOF-GA-2012-332045). B.Y. was supported by the MBI in Singapore. M.P.S. was partially supported by the Mechanobiology Institute, National University of Singapore.
Author information
Authors and Affiliations
Contributions
M.S., S.L., B.Y., R.C. and J.H., performed the experiments; S.L. wrote MATLAB codes for data analysis; M.S., S.L., B.Y., C.H. and R.C. analysed the data; H.W., J.H., and M.P.S. designed the study; M.S., S.L., H.W., J.H. and M.P.S. wrote and prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2360 kb)
Rights and permissions
About this article
Cite this article
Saxena, M., Liu, S., Yang, B. et al. EGFR and HER2 activate rigidity sensing only on rigid matrices. Nature Mater 16, 775–781 (2017). https://doi.org/10.1038/nmat4893
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4893
This article is cited by
-
Monolayer culture alters EGFR inhibitor response through abrogation of microRNA-mediated feedback regulation
Scientific Reports (2024)
-
Emerging roles and mechanisms of ERK pathway mechanosensing
Cellular and Molecular Life Sciences (2023)
-
Probing mechanobiological role of filamin A in migration and invasion of human U87 glioblastoma cells using submicron soft pillars
Nano Convergence (2021)
-
Stopping transformed cancer cell growth by rigidity sensing
Nature Materials (2020)
-
EGFR as a stable marker of prostate cancer dissemination to bones
British Journal of Cancer (2020)