Abstract
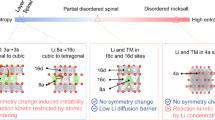
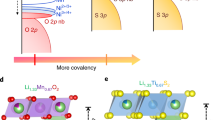
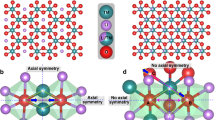
Lithium-ion battery cathode materials have relied on cationic redox reactions until the recent discovery of anionic redox activity in Li-rich layered compounds which enables capacities as high as 300 mAh g−1. In the quest for new high-capacity electrodes with anionic redox, a still unanswered question was remaining regarding the importance of the structural dimensionality. The present manuscript provides an answer. We herein report on a β-Li2IrO3 phase which, in spite of having the Ir arranged in a tridimensional (3D) framework instead of the typical two-dimensional (2D) layers seen in other Li-rich oxides, can reversibly exchange 2.5 e− per Ir, the highest value ever reported for any insertion reaction involving d-metals. We show that such a large activity results from joint reversible cationic (Mn+) and anionic (O2)n− redox processes, the latter being visualized via complementary transmission electron microscopy and neutron diffraction experiments, and confirmed by density functional theory calculations. Moreover, β-Li2IrO3 presents a good cycling behaviour while showing neither cationic migration nor shearing of atomic layers as seen in 2D-layered Li-rich materials. Remarkably, the anionic redox process occurs jointly with the oxidation of Ir4+ at potentials as low as 3.4 V versus Li+/Li0, as equivalently observed in the layered α-Li2IrO3 polymorph. Theoretical calculations elucidate the electrochemical similarities and differences of the 3D versus 2D polymorphs in terms of structural, electronic and mechanical descriptors. Our findings free the structural dimensionality constraint and broaden the possibilities in designing high-energy-density electrodes for the next generation of Li-ion batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997).
Masquelier, C. & Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 113, 6552–6591 (2013).
Rousse, G. & Tarascon, J. M. Sulfate-based polyanionic compounds for Li-Ion batteries: synthesis, crystal chemistry, and electrochemistry aspects. Chem. Mater. 26, 394–406 (2014).
Lu, Z. H., MacNeil, D. D. & Dahn, J. R. Layered LiNixCo1−2xMnxO2 cathode materials for lithium-ion batteries. Electrochem. Solid State Lett. 4, A200–A203 (2001).
Lu, Z., Beaulieu, L. Y., Donaberger, R. A., Thomas, C. L. & Dahn, J. R. Synthesis, structure, and electrochemical behavior of Li[NixLi1/3−2x/3Mn2/3−x/3]O2 . J. Electrochem. Soc. 149, A778–A791 (2002).
Johnson, C. S. et al. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3. (1-x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 6, 1085–1091 (2004).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Sathiya, M. et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 14, 230–238 (2015).
McCalla, E. et al. Visualization of O-O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Takayama, T. et al. Hyperhoneycomb Iridate β-Li2IrO3 as a platform for Kitaev magnetism. Phys. Rev. Lett. 114, 077202 (2015).
Biffin, A. et al. Unconventional magnetic order on the hyperhoneycomb Kitaev lattice in β-Li2IrO3: full solution via magnetic resonant X-ray diffraction. Phys. Rev. B 90, 205116 (2014).
Modic, K. A. et al. Realization of a three-dimensional spin–anisotropic harmonic honeycomb iridate. Nat. Commun. 5, 4203 (2014).
Kimchi, I., Analytis, J. G. & Vishwanath, A. Three-dimensional quantum spin liquids in models of harmonic-honeycomb iridates and phase diagram in an infinite- D approximation. Phys. Rev. B 90, 205126 (2014).
Kimchi, I., Coldea, R. & Vishwanath, A. Unified theory of spiral magnetism in the harmonic-honeycomb iridates α, β, and γ Li2IrO3 . Phys. Rev. B 91, 245134 (2015).
Thackeray, M. M. et al. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112–3125 (2007).
Shannon, R. D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 32, 751–767 (1976).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Freire, M. et al. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 15, 173–177 (2015).
Rouxel, J. Design and chemical reactivity of low dimensional solids—some soft chemistry routes to new solids. Acs Symp. Ser. 499, 88–113 (1992).
Rouxel, J. Anion–cation redox competition and the formation of new compounds in highly covalent systems. Chem.-Eur. J. 2, 1053–1059 (1996).
Rouxel, J. The importance of anions in redox-type chimie douce. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A 310, 1–4 (1998).
Shirley, D. A. High-resolution X-Ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 5, 4709–4714 (1972).
Scofield, J. H. Hartree–Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 8, 129–137 (1976).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics for liquid-metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA + U study. Phys. Rev. B 57, 1505–1509 (1998).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Marmier, A. et al. ElAM: a computer program for the analysis and representation of anisotropic elastic properties. Comput. Phys. Commun. 181, 2102–2115 (2010).
Acknowledgements
The authors thank Q. Jacquet for fruitful discussions and V. Pomjakushin for his valuable help in neutron diffraction experiments. This work is based on experiments performed at the Swiss Spallation Neutron Source SINQ, Paul Scherrer Institute, Villigen, Switzerland. Use of the 11-BM mail service of the APS at Argonne National Laboratory was supported by the US Department of Energy under contract No. DE-AC02-06CH11357 and is greatly acknowledged. J.-M.T. acknowledges funding from the European Research Council (ERC) (FP/2014)/ERC Grant-Project 670116-ARPEMA. E.M. acknowledges financial support from the Fonds de Recherche du Québec—Nature et Technologies.
Author information
Authors and Affiliations
Contributions
P.E.P. and J.-M.T. carried out the synthesis; P.E.P., A.J.P. and J.-M.T. did the electrochemical work; E.M. and G.R. conducted the structural analysis; M.S. and M.-L.D. did the DFT calculations; D.B., A.M.A. and G.V.T. did the TEM study; D.F. collected and analysed the XPS spectra; G.R. and J.-M.T. wrote the manuscript and all authors discussed the experiments and final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4700 kb)
Supplementary Information
Supplementary Information (CIF 1 kb)
Supplementary Information
Supplementary Information (CIF 1 kb)
Rights and permissions
About this article
Cite this article
Pearce, P., Perez, A., Rousse, G. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nature Mater 16, 580–586 (2017). https://doi.org/10.1038/nmat4864
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4864
This article is cited by
-
Understanding voltage hysteresis and decay during anionic redox reaction in layered transition metal oxide cathodes: A critical review
Nano Research (2023)
-
In-situ study for the elastic structure evolutions of three-dimensional Ir-O framework during the oxygen evolution reaction in acid
Nano Research (2023)
-
Interfacial reactions in lithia-based cathodes depending on the binder in the electrode and salt in the electrolyte
Scientific Reports (2022)
-
Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries
Nature Communications (2022)
-
Origin of structural degradation in Li-rich layered oxide cathode
Nature (2022)