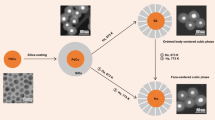
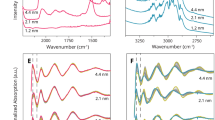
Abstract
Bimetallic, nanostructured materials hold promise for improving catalyst activity and selectivity, yet little is known about the dynamic compositional and structural changes that these systems undergo during pretreatment that leads to efficient catalyst function. Here we use ozone-activated silver–gold alloys in the form of nanoporous gold as a case study to demonstrate the dynamic behaviour of bimetallic systems during activation to produce a functioning catalyst. We show that it is these dynamic changes that give rise to the observed catalytic activity. Advanced in situ electron microscopy and X-ray photoelectron spectroscopy are used to demonstrate that major restructuring and compositional changes occur along the path to catalytic function for selective alcohol oxidation. Transient kinetic measurements correlate the restructuring to three types of oxygen on the surface. The direct influence of changes in surface silver concentration and restructuring at the nanoscale on oxidation activity is demonstrated. Our results demonstrate that characterization of these dynamic changes is necessary to unlock the full potential of bimetallic catalytic materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Xin, H. L. et al. Revealing the atomic restructuring of Pt–Co nanoparticles. Nano Lett. 14, 3203–3207 (2014).
Tao, F. et al. Reaction-driven restructuring of Rh–Pd and Pt–Pd core–shell nanoparticles. Science 322, 932–934 (2008).
Brett, G. L. et al. Selective oxidation of glycerol by highly active bimetallic catalysts at ambient temperature under base-free conditions. Angew. Chem. 123, 10318–10321 (2011).
Della Pina, C., Falletta, E. & Rossi, M. Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold–copper catalyst. J. Catalys. 260, 384–386 (2008).
Whiting, G. T. et al. Methyl formate formation from methanol oxidation using supported gold–palladium nanoparticles. ACS Catalys. 5, 637–644 (2015).
Kotionova, T. et al. Oxidative esterification of homologous 1,3-propanediols. Catalys. Lett. 142, 1114–1120 (2012).
Suzuki, K. et al. Aerobic oxidative esterification of aldehydes with alcohols by gold–nickel oxide nanoparticle catalysts with a core–shell structure. ACS Catalys. 3, 1845–1849 (2013).
Stowers, K. J., Madix, R. J. & Friend, C. M. From model studies on Au(111) to working conditions with unsupported nanoporous gold catalysts: oxygen-assisted coupling reactions. J. Catalys. 308, 131–141 (2013).
Wang, L.-C. et al. Exploiting basic principles to control the selectivity of the vapor phase catalytic oxidative cross-coupling of primary alcohols over nanoporous gold catalysts. J. Catalys. 329, 78–86 (2015).
Sault, A. G., Madix, R. J. & Campbell, C. T. Adsorption of oxygen and hydrogen on Au(110)-(1 × 2). Surf. Sci. 169, 347–356 (1986).
Meyer, R., Lemire, C., Shaikhutdinov, S. K. & Freund, H. J. Surface chemistry of catalysis by gold. Gold Bull. 37, 72–124 (2011).
Personick, M. L. et al. Ozone-activated nanoporous gold: a stable and storable material for catalytic oxidation. ACS Catalys. 5, 4237–4241 (2015).
Xu, C. et al. Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 129, 42–43 (2007).
Biener, M. M. et al. ALD functionalized nanoporous gold: thermal stability, mechanical properties, and catalytic activity. Nano Lett. 11, 3085–3090 (2011).
Fujita, T. et al. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 11, 775–780 (2012).
Xu, B., Liu, X., Haubrich, J., Madix, R. J. & Friend, C. M. Selectivity control in gold-mediated esterification of methanol. Angew. Chem. Int. Ed. 48, 4206–4209 (2009).
Xu, B., Haubrich, J., Freyschlag, C. G., Madix, R. J. & Friend, C. M. Oxygen-assisted cross-coupling of methanol with alkyl alcohols on metallic gold. Chem. Sci. 1, 310–316 (2010).
Wang, L. C. et al. Active sites for methanol partial oxidation on nanoporous gold catalysts. J. Catalys. 344, 778–783 (2016).
in Non-tetrahedrally Bonded Elements and Binary Compounds I Vol. 41C (eds Madelung, O., Rössler, U. & Schulz, M.) 1–3 (Springer, 1998).
Jones, P. G. et al. Gold(III) oxide. Acta Crystallogr. B 35, 1435–1437 (1979).
Krozer, A. & Rodahl, M. X-ray photoemission spectroscopy study of UV/ozone oxidation of Au under ultrahigh vacuum conditions. J. Vac. Sci. Technol. A 15, 1704–1707 (1997).
Tanuma, S., Powell, C. J. & Penn, D. R. Calculations of electron inelastic mean free paths. Surf. Interface Anal. 21, 165–176 (1994).
Kaspar, T. C., Droubay, T., Chambers, S. A. & Bagus, P. S. Spectroscopic evidence for Ag(III) in highly oxidized silver films by X-ray photoelectron spectroscopy. J. Phys. Chem. C 114, 21562–21571 (2010).
Hammond, J. S. & Gaarenstroom, S. W. X-ray photoelectron spectroscopic studies of cadmium– and silver–oxygen surfaces. Anal. Chem. 47, 2193–2199 (1975).
Hoflund, G. B., Hazos, Z. F. & Salaita, G. N. Surface characterization study of Ag, AgO, and Ag2O using x-ray photoelectron spectroscopy and electron energy-loss spectroscopy. Phys. Rev. B 62, 11126–11133 (2000).
Xu, B., Siler, C. G. F., Madix, R. J. & Friend, C. M. Ag/Au mixed sites promote oxidative coupling of methanol on the alloy surface. Chem. Eur. J. 20, 4646–4652 (2014).
Boronin, A. I., Koscheev, S. V. & Zhidomirov, G. M. XPS and UPS study of oxygen states on silver. J. Electron Spectrosc. Relat. Phenom. 96, 43–51 (1998).
Tyson, C. C. et al. Charge redistribution in Au–Ag alloys from a local perspective. Phys. Rev. B 45, 8924–8928 (1992).
Kibis, L. S. et al. The investigation of oxidized silver nanoparticles prepared by thermal evaporation and radio-frequency sputtering of metallic silver under oxygen. Appl. Surf. Sci. 257, 404–413 (2010).
Moskaleva, L. V., Weiss, T., Klüner, T. & Bäumer, M. Chemisorbed oxygen on the Au(321) surface alloyed with silver: a first-principles investigation. J. Phys. Chem. C 119, 9215–9226 (2015).
Fujita, T. et al. Atomic observation of catalysis-induced nanopore coarsening of nanoporous gold. Nano Lett. 14, 1172–1177 (2014).
Acknowledgements
This work was supported as part of the Integrated Mesoscale Architectures for Sustainable Catalysis, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award no. DE-SC0012573. Work at LLNL was performed under the auspices of the US Department of Energy by LLNL under Contract DE-AC52-07NA27344. This research used resources of the Center for Functional Nanomaterials, which is a US DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. It also used resources of the Advance Light Source, which is supported by the Office of Science of the US DOE under Contract No. DE-AC02-05CH11231. We thank H. Bluhm for advice on the preparation of the AP-XPS experiments and H. Xin for valuable discussion regarding the EELS data.
Author information
Authors and Affiliations
Contributions
C.M.F., R.J.M., J.B. and B.Z. conceived and designed the experimental plan. B.Z. conducted the flow reactor experiments. B.Z. and J.B. prepared the npAu samples. L.W. conceived and conducted the TAP reactor experiments. C.H., M.S., B.A.J.L. and B.Z. conceived and conducted the AP-XPS experiments and analysed the XPS data. E.A.S., D.N.Z. and B.Z. planned and conducted E-TEM experiments. B.Z. wrote the initial draft of the manuscript. All authors discussed the results and contributed to the final preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1325 kb)
Rights and permissions
About this article
Cite this article
Zugic, B., Wang, L., Heine, C. et al. Dynamic restructuring drives catalytic activity on nanoporous gold–silver alloy catalysts. Nature Mater 16, 558–564 (2017). https://doi.org/10.1038/nmat4824
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4824
This article is cited by
-
Strategies to improve hydrogen activation on gold catalysts
Nature Reviews Chemistry (2024)
-
Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction
Nature Catalysis (2024)
-
Time-resolved transmission electron microscopy for nanoscale chemical dynamics
Nature Reviews Chemistry (2023)
-
Structural and optical properties of gold nanosponges revealed via 3D nano-reconstruction and phase-field models
Communications Materials (2023)
-
Influence of framework Al density in chabazite zeolites on copper ion mobility and reactivity during NOx selective catalytic reduction with NH3
Nature Catalysis (2023)