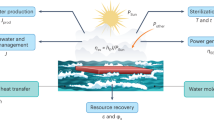
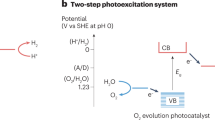
Abstract
The generation of hydrogen from water and sunlight offers a promising approach for producing scalable and sustainable carbon-free energy. The key of a successful solar-to-fuel technology is the design of efficient, long-lasting and low-cost photoelectrochemical cells, which are responsible for absorbing sunlight and driving water splitting reactions. To this end, a detailed understanding and control of heterogeneous interfaces between photoabsorbers, electrolytes and catalysts present in photoelectrochemical cells is essential. Here we review recent progress and open challenges in predicting physicochemical properties of heterogeneous interfaces for solar water splitting applications using first-principles-based approaches, and highlights the key role of these calculations in interpreting increasingly complex experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
McKone, J. R., Lewis, N. S. & Gray, H.B. Will solar-driven water-splitting devices see the light of day? Chem. Mater. 26, 407–414 (2013).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Chen, X., Shen, S., Guo, L. & Mao, S. S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110, 6503–6570 (2010).
Esposito, D. V. et al. Methods of photoelectrode characterization with high spatial and temporal resolution. Energy Environ. Sci. 8, 2863–2885 (2015).
Hydrogen and Fuel Cells Program Plan (Department of Energy, 2011).
Luo, J. et al. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 345, 1593–1596 (2014).
Abdi, F. F. et al. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 4, 1–7 (2013).
Coridan, R. H., Shaner, M., Wiggenhorn, C., Brunschwig, B. S. & Lewis, N. S. Electrical and photoelectrochemical properties of WO3/Si tandem photoelectrodes. J. Phys. Chem. C 117, 6949–6957 (2013).
Lichterman, M. F. et al. Stabilization of n-cadmium telluride photoanodes for water oxidation to O2 (g) in aqueous alkaline electrolytes using amorphous TiO2 films formed by atomic-layer deposition. Energy Environ. Sci. 7, 3334–3337 (2014).
Benck, J. D. et al. Designing active and stable silicon photocathodes for solar hydrogen production using molybdenum sulfide nanomaterials. Adv. Energy Mater. 4, 1400739 (2014).
Kim, T. W. & Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Hu, S. et al. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 344, 1005–1009 (2014).
May, M. M., Lewerenz, H.-J., Lackner, D., Dimroth, F. & Hannappel, T. Efficient direct solar-to-hydrogen conversion by in situ interface transformation of a tandem structure. Nat. Commun. 6, 8286 (2015).
Smith, W. A., Sharp, I. D., Strandwitz, N. C. & Bisquert, J. Interfacial band-edge energetics for solar fuels production. Energy Environ. Sci. 8, 2851–2862 (2015).
Chen, S. & Wang, L.-W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 24, 3659–3666 (2012).
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. & Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006).
Jain, A., Castelli, I. E., Hautier, G., Bailey, D. H. & Jacobsen, K. W. Performance of genetic algorithms in search for water splitting perovskites. J. Mater. Sci. 48, 6519–6534 (2013).
Castelli, I. E. et al. New light-harvesting materials using accurate and efficient bandgap calculations. Adv. Energy Mater. 5, 1400915 (2015).
Liao, P. & Carter, E. A. New concepts and modeling strategies to design and evaluate photo-electro-catalysts based on transition metal oxides. Chem. Soc. Rev. 42, 2401–2422 (2013).
Bhatt, M. D. & Lee, J. S. Recent theoretical progress in the development of photoanode materials for solar water splitting photoelectrochemical cells. J. Mater. Chem. A 3, 10632–10659 (2015).
Burke, K. Perspective on density functional theory. J. Chem. Phys. 136, 150901 (2012).
Vignale, G. & Rasolt, M. Density-functional theory in strong magnetic fields. Phys. Rev. Lett. 59, 2360 (1987).
Car, R. & Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 55, 2471 (1985).
Sulpizi, M., Salanne, M., Sprik, M. & Gaigeot, M.-P. Vibrational sum frequency generation spectroscopy of the water liquid–vapor interface from density functional theory-based molecular dynamics simulations. J. Phys. Chem. Lett. 4, 83–87 (2012).
Huang, P., Pham, T. A., Galli, G. & Schwegler, E. Alumina (0001)/water interface: structural properties and infrared spectra from first-principles molecular dynamics simulations. J. Phys. Chem. C 118, 8944–8951 (2014).
Prendergast, D. & Galli, G. X-ray absorption spectra of water from first principles calculations. Phys. Rev. Lett. 96, 215502 (2006).
Velasco-Velez, J.-J. et al. The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy. Science 346, 831–834 (2014).
Cohen, A. J., Mori-Sánchez, P. & Yang, W. Challenges for density functional theory. Chem. Rev. 112, 289–320 (2011).
Gillan, M. J., Alfè, D. & Michaelides, A. Perspective: how good is DFT for water? J. Chem. Phys. 144, 130901 (2016).
Ping, Y., Rocca, D. & Galli, G. Electronic excitations in light absorbers for photoelectrochemical energy conversion: first principles calculations based on many body perturbation theory. Chem. Soc. Rev. 42, 2437–2469 (2013).
Grossman, J. C., Schwegler, E., Draeger, E. W., Gygi, F. & Galli, G. Towards an assessment of the accuracy of density functional theory for first principles simulations of water. J. Chem. Phys. 120, 300–311 (2004).
Schwegler, E., Grossman, J. C., Gygi, F. & Galli, G. Towards an assessment of the accuracy of density functional theory for first principles simulations of water. II. J. Chem. Phys. 121, 5400–5409 (2004).
Zhang, C., Donadio, D., Gygi, F. & Galli, G. First principles simulations of the infrared spectrum of liquid water using hybrid density functionals. J. Chem. Theory Comput. 7, 1443–1449 (2011).
Wan, Q., Spanu, L., Gygi, F. & Galli, G. Electronic structure of aqueous sulfuric acid from first-principles simulations with hybrid functionals. J. Phys. Chem. Lett. 5, 2562–2567 (2014).
Zhang, C., Pham, T. A., Gygi, F. & Galli, G. Electronic structure of the solvated chloride anion from first principles molecular dynamics. J. Chem. Phys. 138, 181102 (2013).
Gaiduk, A. P., Zhang, C., Gygi, F. & Galli, G. Structural and electronic properties of aqueous NaCl solutions from ab initio molecular dynamics simulations with hybrid density functionals. Chem. Phys. Lett. 604, 89–96 (2014).
DiStasio, R. A. Jr, Santra, B., Li, Z., Wu, X. & Car, R. The individual and collective effects of exact exchange and dispersion interactions on the ab initio structure of liquid water. J. Chem. Phys. 141, 084502 (2014).
Wang, J., Román-Pérez, G., Soler, J. M., Artacho, E. & Fernández-Serra, M.-V. Density, structure, and dynamics of water: the effect of van der Waals interactions. J. Chem. Phys. 134, 024516 (2011).
Wróbel, J., Kurzydłowski, K. J., Hummer, K., Kresse, G. & Piechota, J. Calculations of ZnO properties using the Heyd-Scuseria-Ernzerhof screened hybrid density functional. Phys. Rev. B 80, 155124 (2009).
Janotti, A. et al. Hybrid functional studies of the oxygen vacancy in TiO2 . Phys. Rev. B 81, 085212 (2010).
Kweon, K. E. & Hwang, G. S. Structural phase-dependent hole localization and transport in bismuth vanadate. Phys. Rev. B 87, 205202 (2013).
Ping, Y., Goddard, W. A. III & Galli, G. A. Energetics and solvation effects at the photoanode/catalyst interface: ohmic contact versus Schottky barrier. J. Am. Chem. Soc. 137, 5264–5267 (2015).
Ataca, C., Sahin, H. & Ciraci, S. Stable, single-layer MX2 transition-metal oxides and dichalcogenides in a honeycomb-like structure. J. Phys. Chem. C 116, 8983–8999 (2012).
Niu, P., Zhang, L., Liu, G. & Cheng, H.-M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Energy Mater. 22, 4763–4770 (2012).
Singh, A. K., Mathew, K., Zhuang, H. L. & Hennig, R. G. Computational screening of 2D materials for photocatalysis. J. Phys. Chem. Lett. 6, 1087–1098 (2015).
Cheng, J., VandeVondele, J. & Sprik, M. Identifying trapped electronic holes at the aqueous TiO2 interface. J. Phys. Chem. C 118, 5437–5444 (2014).
Dawson, W. & Gygi, F. Performance and accuracy of recursive subspace bisection for hybrid DFT calculations in inhomogeneous systems. J. Chem. Theory Comput. 11, 4655–4663 (2015).
Zhang, C., Wu, J., Galli, G. & Gygi, F. Structural and vibrational properties of liquid water from van der Waals density functionals. J. Chem. Theory Comput. 7, 3054–3061 (2011).
Marsman, M., Paier, J., Stroppa, A. & Kresse, G. Hybrid functionals applied to extended systems. J. Phys. Condens. Matter 20, 064201 (2008).
Anisimov, V. I., Aryasetiawan, F. & Lichtenstein, A. First-principles calculations of the electronic structure and spectra of strongly correlated systems: the LDA + U method. J. Phys. Condens. Matter 9, 767 (1997).
Skone, J. H., Govoni, M. & Galli, G. Self-consistent hybrid functional for condensed systems. Phys. Rev. B 89, 195112 (2014).
Kulik, H. J., Cococcioni, M., Scherlis, D. A. & Marzari, N. Density functional theory in transition-metal chemistry: a self-consistent Hubbard U approach. Phys. Rev. Lett. 97, 103001 (2006).
Mosey, N. J., Liao, P. & Carter, E. A. Rotationally invariant ab initio evaluation of Coulomb and exchange parameters for DFT + U calculations. J. Chem. Phys. 129, 014103 (2008).
Hybertsen, M. S. & Louie, S. G. First-principles theory of quasiparticles: calculation of band gaps in semiconductors and insulators. Phys. Rev. Lett. 55, 1418–1421 (1985).
Pham, T. A. et al. Band offset and dielectric properties of the amorphous Si3N4/Si(001) interface: a first-principles study. Appl. Phys. Lett. 102, 241603 (2013).
Pham, T. A., Zhang, C., Schwegler, E. & Galli, G. Probing the electronic structure of liquid water with many-body perturbation theory. Phys. Rev. B 89, 060202 (2014).
Opalka, D., Pham, T. A., Sprik, M. & Galli, G. The ionization potential of aqueous hydroxide computed using many-body perturbation theory. J. Chem. Phys. 141, 034501 (2014).
Opalka, D., Pham, T. A., Galli, G. & Sprik, M. Electronic energy levels and band alignment for aqueous phenol and phenolate from first principles. J. Phys. Chem. B 119, 9651–9660 (2015).
Nguyen, H.-V., Pham, T. A., Rocca, D. & Galli, G. Improving accuracy and efficiency of calculations of photoemission spectra within many-body perturbation theory. Phys. Rev. B 85, 081101 (2012).
Pham, T. A., Nguyen, H.-V., Rocca, D. & Galli, G. GW calculations using the spectral decomposition of the dielectric matrix: verification, validation, and comparison of methods. Phys. Rev. B 87, 155148 (2013).
Pham, T. A., Lee, D., Schwegler, E. & Galli, G. Interfacial effects on the band edges of functionalized Si surfaces in liquid water. J. Am. Chem. Soc. 136, 17071–17077 (2014).
Govoni, M. & Galli, G. Large scale GW calculations. J. Chem. Theory Comput. 11, 2680–2696 (2015).
Cheng, J. & Sprik, M. Aligning electronic energy levels at the TiO2/H2O interface. Phys. Rev. B 82, 081406 (2010).
Wood, B. C., Schwegler, E., Choi, W. I. & Ogitsu, T. Hydrogen-bond dynamics of water at the interface with InP/GaP (001) and the implications for photoelectrochemistry. J. Am. Chem. Soc. 135, 15774–15783 (2013).
Wood, B. C., Schwegler, E., Choi, W. I. & Ogitsu, T. Surface chemistry of GaP (001) and InP (001) in contact with water. J. Phys. Chem. C 118, 1062–1070 (2014).
Shen, X. et al. Photocatalytic water oxidation at the GaN (101IE0)- water interface. J. Phys. Chem. C 114, 13695–13704 (2010).
Wang, J., Pedroza, L. S., Poissier, A. & Fernández-Serra, M. Water dissociation at the GaN (10 0) surface: structure, dynamics and surface acidity. J. Phys. Chem. C 116, 14382–14389 (2012).
Kharche, N., Muckerman, J. T. & Hybertsen, M. S. First-principles approach to calculating energy level alignment at aqueous semiconductor interfaces. Phys. Rev. Lett. 113, 176802 (2014).
English, N. J., Rahman, M., Wadnerkar, N. & MacElroy, J. Photo-active and dynamical properties of hematite (Fe2O3)–water interfaces: an experimental and theoretical study. Phys. Chem. Chem. Phys. 16, 14445–14454 (2014).
Sun, C., Liu, L.-M., Selloni, A., Lu, G. Q. M. & Smith, S. C. Titania-water interactions: a review of theoretical studies. J. Mater. Chem. 20, 10319–10334 (2010).
Liu, L.-M., Zhang, C., Thornton, G. & Michaelides, A. Structure and dynamics of liquid water on rutile TiO2 (110). Phys. Rev. B 82, 161415 (2010).
Duncan, D. A., Allegretti, F. & Woodruff, D. Water does partially dissociate on the perfect TiO2 (110) surface: a quantitative structure determination. Phys. Rev. B 86, 045411 (2012).
Kumar, N., Kent, P. R., Wesolowski, D. J. & Kubicki, J. D. Modeling water adsorption on rutile (110) using van der Waals density functional and DFT + U methods. J. Phys. Chem. C 117, 23638–23644 (2013).
De Angelis, F., Di Valentin, C., Fantacci, S., Vittadini, A. & Selloni, A. Theoretical studies on anatase and less common TiO2 phases: bulk, surfaces, and nanomaterials. Chem. Rev. 114, 9708–9753 (2014).
Allegretti, F., O’Brien, S., Polcik, M., Sayago, D. I. & Woodruff, D. P. Adsorption bond length for H2O on TiO2 (110): a key parameter for theoretical understanding. Phys. Rev. Lett. 95, 226104 (2005).
Allegretti, F., O’Brien, S., Polcik, M., Sayago, D. I. & Woodruff, D. P. Quantitative determination of the local structure of H2O on TiO2 (110) using scanned-energy mode photoelectron diffraction. Surf. Sci. 600, 1487–1496 (2006).
Walle, L., Borg, A., Uvdal, P. & Sandell, A. Experimental evidence for mixed dissociative and molecular adsorption of water on a rutile TiO2 (110) surface without oxygen vacancies. Phys. Rev. B 80, 235436 (2009).
Hahn, K. R., Tricoli, A., Santarossa, G., Vargas, A. & Baiker, A. First principles analysis of H2O adsorption on the (110) surfaces of SnO2, TiO2 and their solid solutions. Langmuir 28, 1646–1656 (2011).
Lindan, P. J., Harrison, N. & Gillan, M. Mixed dissociative and molecular adsorption of water on the rutile (110) surface. Phys. Rev. Lett. 80, 762 (1998).
Zhang, C. & Lindan, P. J. Multilayer water adsorption on rutile TiO2 (110): a first-principles study. J. Chem. Phys. 118, 4620–4630 (2003).
Kumar, N. et al. Hydrogen bonds and vibrations of water on (110) rutile. J. Phys. Chem. C 113, 13732–13740 (2009).
Harris, L. A. & Quong, A. A. Molecular chemisorption as the theoretically preferred pathway for water adsorption on ideal rutile TiO2 (110). Phys. Rev. Lett. 93, 086105 (2004).
Wan, Q. & Galli, G. First-principles framework to compute sum-frequency generation vibrational spectra of semiconductors and insulators. Phys. Rev. Lett. 115, 246404 (2015).
Toroker, M. C. et al. First principles scheme to evaluate band edge positions in potential transition metal oxide photocatalysts and photoelectrodes. Phys. Chem. Chem. Phys. 13, 16644–16654 (2011).
Jiang, H. Electronic band structures of molybdenum and tungsten dichalcogenides by the GW approach. J. Phys. Chem. C 116, 7664–7671 (2012).
Stevanović, V., Lany, S., Ginley, D. S., Tumas, W. & Zunger, A. Assessing capability of semiconductors to split water using ionization potentials and electron affinities only. Phys. Chem. Chem. Phys. 16, 3706–3714 (2014).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Cheng, J. & Sprik, M. Alignment of electronic energy levels at electrochemical interfaces. Phys. Chem. Chem. Phys. 14, 11245–11267 (2012).
Wu, Y., Chan, M. K. Y. & Ceder, G. Prediction of semiconductor band edge positions in aqueous environments from first principles. Phys. Rev. B 83, 235301 (2011).
Li, Y., O’Leary, L. E., Lewis, N. S. & Galli, G. Combined theoretical and experimental study of band-edge control of Si through surface functionalization. J. Phys. Chem. C 117, 5188–5194 (2013).
Ping, Y., Sundararaman, R. & Goddard, W. A. III Solvation effects on the band edge positions of photocatalysts from first principles. Phys. Chem. Chem. Phys. 17, 30499–30509 (2015).
McCrory, C. C. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015).
Spurgeon, J. M., Velazquez, J. M. & McDowell, M. T. Improving O2 production of WO3 photoanodes with IrO2 in acidic aqueous electrolyte. Phys. Chem. Chem. Phys. 16, 3623–3631 (2014).
Sundararaman, R., Schwarz, K. A., Letchworth-Weaver, K. & Arias, T. Spicing up continuum solvation models with SaLSA: the spherically averaged liquid susceptibility ansatz. J. Chem. Phys. 142, 054102 (2015).
Sundararaman, R. & Goddard, W. A. III The charge-asymmetric nonlocally determined local-electric (CANDLE) solvation model. J. Chem. Phys. 142, 064107 (2015).
Kim, T. W., Ping, Y., Galli, G. A. & Choi, K.-S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 6, 8769 (2015).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Fang, Y.-H. & Liu, Z.-P. Mechanism and tafel lines of electro-oxidation of water to oxygen on RuO2 (110). J. Am. Chem. Soc. 132, 18214–18222 (2010).
Huang, Y., Nielsen, R. J., Goddard, W. A. III & Soriaga, M. P. The reaction mechanism with free energy barriers for electrochemical dihydrogen evolution on MoS2 . J. Am. Chem. Soc. 137, 6692–6698 (2015).
Xiao, H., Cheng, T., Goddard, W. A. III & Sundararaman, R. Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu (111). J. Am. Chem. Soc. 138, 483–486 (2016).
Letchworth-Weaver, K. & Arias, T. Joint density functional theory of the electrode-electrolyte interface: application to fixed electrode potentials, interfacial capacitances, and potentials of zero charge. Phys. Rev. B 86, 075140 (2012).
Gunceler, D., Letchworth-Weaver, K., Sundararaman, R., Schwarz, K. A. & Arias, T. The importance of nonlinear fluid response in joint density-functional theory studies of battery systems. Model. Simul. Mater. Sci. 21, 074005 (2013).
Atalla, V., Yoon, M., Caruso, F., Rinke, P. & Scheffler, M. Hybrid density functional theory meets quasiparticle calculations: a consistent electronic structure approach. Phys. Rev. B 88, 165122 (2013).
Cheng, H. & Selloni, A. Hydroxide ions at the water/anatase TiO2 (101) interface: structure and electronic states from first principles molecular dynamics. Langmuir 26, 11518–11525 (2010).
Cheng, J. & Sprik, M. The electric double layer at a rutile TiO2 water interface modelled using density functional theory based molecular dynamics simulation. J. Phys. Condens. Matter 26, 244108 (2014).
Laio, A. & Gervasio, F. L. Metadynamics: a method to simulate rare events and reconstruct the free energy in biophysics, chemistry and material science. Rep. Prog. Phys. 71, 126601 (2008).
Otani, M. & Sugino, O. First-principles calculations of charged surfaces and interfaces: a plane-wave nonrepeated slab approach. Phys. Rev. B 73, 115407 (2006).
Bonnet, N., Morishita, T., Sugino, O. & Otani, M. First-principles molecular dynamics at a constant electrode potential. Phys. Rev. Lett. 109, 266101 (2012).
Gaiduk, A. P. et al. Photoelectron spectra of aqueous solutions from first principles. J. Am. Chem. Soc. 138, 6912–6915 (2016).
Morbec, J. M., Narkeviciute, I., Jaramillo, T. F. & Galli, G. Optoelectronic properties of Ta3N5: a joint theoretical and experimental study. Phys. Rev. B 90, 155204 (2014).
Akimov, A. V., Neukirch, A. J. & Prezhdo, O. V. Theoretical insights into photoinduced charge transfer and catalysis at oxide interfaces. Chem. Rev. 113, 4496–4565 (2013).
Pastore, M. & Angelis, F. D. First-principles modeling of a dye-sensitized TiO2/IrO2 photoanode for water oxidation. J. Am. Chem. Soc. 137, 5798–5809 (2015).
Acknowledgements
This work was supported by the NSF-CCI grant (CHE-1305124). Part of this work was performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. T.A.P. acknowledges support from the Lawrence Fellowship. We thank B. Wood, T. Ogitsu and E. Schwegler for useful discussions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the discussion and writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pham, T., Ping, Y. & Galli, G. Modelling heterogeneous interfaces for solar water splitting. Nature Mater 16, 401–408 (2017). https://doi.org/10.1038/nmat4803
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4803
This article is cited by
-
In situ electrochemical Raman spectroscopy and ab initio molecular dynamics study of interfacial water on a single-crystal surface
Nature Protocols (2023)
-
Accurate quantification of the stability of the perylene-tetracarboxylic dianhydride on Au(111) molecule–surface interface
Communications Chemistry (2023)
-
Fluctuation-induced quantum friction in nanoscale water flows
Nature (2022)
-
Graphene oxide as a hole extraction layer loaded on BiVO4 photoanode for highly efficient photoelectrochemical water splitting
Rare Metals (2022)
-
Discovering and understanding materials through computation
Nature Materials (2021)