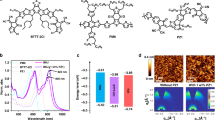
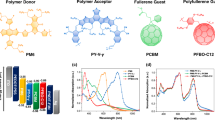
Abstract
Technological deployment of organic photovoltaic modules requires improvements in device light-conversion efficiency and stability while keeping material costs low. Here we demonstrate highly efficient and stable solar cells using a ternary approach, wherein two non-fullerene acceptors are combined with both a scalable and affordable donor polymer, poly(3-hexylthiophene) (P3HT), and a high-efficiency, low-bandgap polymer in a single-layer bulk-heterojunction device. The addition of a strongly absorbing small molecule acceptor into a P3HT-based non-fullerene blend increases the device efficiency up to 7.7 ± 0.1% without any solvent additives. The improvement is assigned to changes in microstructure that reduce charge recombination and increase the photovoltage, and to improved light harvesting across the visible region. The stability of P3HT-based devices in ambient conditions is also significantly improved relative to polymer:fullerene devices. Combined with a low-bandgap donor polymer (PBDTTT-EFT, also known as PCE10), the two mixed acceptors also lead to solar cells with 11.0 ± 0.4% efficiency and a high open-circuit voltage of 1.03 ± 0.01 V.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhao, J. et al. A difluorobenzoxadiazole building block for efficient polymer solar cells. Adv. Mater. 28, 1868–1873 (2016).
Hwang, Y.-J., Li, H., Courtright, B. A. E., Subramaniyan, S. & Jenekhe, S. A. Nonfullerene polymer solar cells with 8.5% efficiency enabled by a new highly twisted electron acceptor dimer. Adv. Mater. 28, 124-131 (2016).
Zhang, J. et al. Conjugated polymer—small molecule alloy leads to high efficient ternary organic solar cells. J. Am. Chem. Soc. 137, 8176–8183 (2015).
Yang, Y. et al. High-performance multiple-donor bulk heterojunction solar cells. Nat. Photon. 9, 190–198 (2015).
Li, N. & Brabec, C. J. Air-processed polymer tandem solar cells with power conversion efficiency exceeding 10%. Energy Environ. Sci. 8, 2902–2909 (2015).
Bannock, J. H. et al. Continuous synthesis of device-grade semiconducting polymers in droplet-based microreactors. Adv. Funct. Mater. 23, 2123–2129 (2013).
Huang, Y.-C. et al. Small- and wide-angle X-ray scattering characterization of bulk heterojunction polymer solar cells with different fullerene derivatives. J. Phys. Chem. C 116, 10238–10244 (2012).
Yin, W. & Dadmun, M. A new model for the morphology of P3HT/PCBM organic photovoltaics from small-angle neutron scattering: rivers and streams. ACS Nano 5, 4756–4768 (2011).
Campoy-Quiles, M. et al. Morphology evolution via self-organization and lateral and vertical diffusion in polymer:fullerene solar cell blends. Nat. Mater. 7, 158–164 (2008).
Dang, M. T., Hirsch, L. & Wantz, G. P3HT:PCBM, Best seller in polymer photovoltaic research. Adv. Mater. 23, 3597–3602 (2011).
Holliday, S. et al. High efficiency and air stable P3HT based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 7, 11585 (2016).
Holliday, S. et al. A rhodanine flanked nonfullerene acceptor for solution-processed organic photovoltaics. J. Am. Chem. Soc. 137, 898–904 (2015).
Sun, D. et al. Non-fullerene-acceptor-based bulk-heterojunction organic solar cells with efficiency over 7%. J. Am. Chem. Soc. 137, 11156–11162 (2015).
Liu, Y. et al. A tetraphenylethylene core-based 3D structure small molecular acceptor enabling efficient non-fullerene organic solar cells. Adv. Mater. 27, 1015–1020 (2015).
Li, H. et al. Beyond fullerenes: design of nonfullerene acceptors for efficient organic photovoltaics. J. Am. Chem. Soc. 136, 14589–14597 (2014).
Meng, D. et al. High-performance solution-processed non-fullerene organic solar cells based on selenophene-containing perylene bisimide acceptor. J. Am. Chem. Soc. 138, 375–380 (2016).
Lin, H. et al. High-performance non-fullerene polymer solar cells based on a pair of donor–acceptor materials with complementary absorption properties. Adv. Mater. 27, 7299–7304 (2015).
Li, S. et al. A spirobifluorene and diketopyrrolopyrrole moieties based non-fullerene acceptor for efficient and thermally stable polymer solar cells with high open-circuit voltage. Energy Environ. Sci. 9, 604–610 (2016).
Khlyabich, P. P., Burkhart, B. & Thompson, B. C. Efficient ternary blend bulk heterojunction solar cells with tunable open-circuit voltage. J. Am. Chem. Soc. 133, 14534–14537 (2011).
Lu, L., Chen, W., Xu, T. & Yu, L. High-performance ternary blend polymer solar cells involving both energy transfer and hole relay processes. Nat. Commun. 6, 7327 (2015).
Ameri, T. et al. Morphology analysis of near IR sensitized polymer/fullerene organic solar cells by implementing low bandgap heteroanalogue C-/Si-PCPDTBT. J. Mater. Chem. A 2, 19461–19472 (2014).
Gasparini, N. et al. An alternative strategy to adjust the recombination mechanism of organic photovoltaics by implementing ternary compounds. Adv. Energy Mater. 5, 1501527 (2015).
Lu, L., Kelly, M. A., You, W. & Yu, L. Status and prospects for ternary organic photovoltaics. Nat. Photon. 9, 491–500 (2015).
Zhang, Y. et al. Synergistic effect of polymer and small molecules for high-performance ternary organic solar cells. Adv. Mater. 27, 1071–1076 (2015).
Khlyabich, P. P., Rudenko, A. E., Thompson, B. C. & Loo, Y.-L. Structural origins for tunable open-circuit voltage in ternary-blend organic solar cells. Adv. Funct. Mater. 25, 5557–5563 (2015).
Ke, L. et al. A series of pyrene-substituted silicon phthalocyanines as near-IR sensitizers in organic ternary solar cells. Adv. Energy Mater. 6, 1502355 (2016).
Ko, S.-J. et al. Improved performance in polymer solar cells using mixed PC61BM/PC71BM acceptors. Adv. Energy Mater. 5, 1401687 (2015).
Cheng, P., Li, Y. & Zhan, X. Efficient ternary blend polymer solar cells with indene-C60 bisadduct as an electron-cascade acceptor. Energy Environ. Sci. 7, 2005–2011 (2014).
Kang, H. et al. Effect of fullerene tris-adducts on the photovoltaic performance of P3HT:fullerene ternary blends. ACS Appl. Mater. Interfaces 5, 4401–4408 (2013).
An, Q. et al. Simultaneous improvement in short circuit current, open circuit voltage, and fill factor of polymer solar cells through ternary strategy. ACS Appl. Mater. Interface 7, 3691–3698 (2015).
Huang, T.-Y. et al. Efficient ternary bulk heterojunction solar cells based on small molecules only. J. Mater. Chem. A 3, 10512–10518 (2015).
Nielsen, C. B., Holliday, S., Chen, H.-Y., Cryer, S. J. & McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 48, 2803–2812 (2015).
Zhao, W. et al. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 28, 4734–4739 (2016).
Setayesh, S., Marsitzky, D. & Müllen, K. Bridging the gap between polyfluorene and ladder-poly-p-phenylene: synthesis and characterization of poly-2,8-indenofluorene. Macromolecules 33, 2016–2020 (2000).
Chou, K. W. et al. Spin-cast bulk heterojunction solar cells: a dynamical investigation. Adv. Mater. 25, 1923–1929 (2013).
Perez, L. A. et al. Solvent additive effects on small molecule crystallization in bulk heterojunction solar cells probed during spin casting. Adv. Mater. 25, 6380–6384 (2013).
Abdelsamie, M. et al. Toward additive-free small-molecule organic solar cells: roles of the donor crystallization pathway and dynamics. Adv. Mater. 27, 7285–7292 (2015).
Rivnay, J., Mannsfeld, S. C. B., Miller, C. E., Salleo, A. & Toney, M. F. Quantitative determination of organic semiconductor microstructure from the molecular to device scale. Chem. Rev. 112, 5488–5519 (2012).
Richter, L. J. et al. In situ morphology studies of the mechanism for solution additive effects on the formation of bulk heterojunction films. Adv. Energy Mater. 5, 1400975 (2015).
Credgington, D. & Durrant, J. R. Insights from transient optoelectronic analyses on the open-circuit voltage of organic solar cells. J. Phys. Chem. Lett. 3, 1465–1478 (2012).
Baran, D. et al. Qualitative analysis of bulk-heterojunction solar cells without device fabrication: an elegant and contactless method. J. Am. Chem. Soc. 136, 10949–10955 (2014).
Vandewal, K., Tvingstedt, K., Gadisa, A., Inganas, O. & Manca, J. V. On the origin of the open-circuit voltage of polymer-fullerene solar cells. Nat. Mater. 8, 904–909 (2009).
Deotare, P. B. et al. Nanoscale transport of charge-transfer states in organic donor–acceptor blends. Nat. Mater. 14, 1130–1134 (2015).
Shuttle, C. G., Hamilton, R., Nelson, J., O’Regan, B. C. & Durrant, J. R. Measurement of charge-density dependence of carrier mobility in an organic semiconductor blend. Adv. Funct. Mater. 20, 698–702 (2010).
Dang, B., He, J., Hu, J. & Zhou, Y. Large improvement in trap level and space charge distribution of polypropylene by enhancing the crystalline–amorphous interface effect in blends. Polym. Int. 65, 371–379 (2016).
Baran, D. et al. Role of polymer fractionation in energetic losses and charge carrier lifetimes of polymer: fullerene solar cells. J. Phys. Chem. C 119, 19668–19673 (2015).
Tremolet de Villers, B. J. et al. Removal of residual diiodooctane improves photostability of high-performance organic solar cell polymers. Chem. Mater. 28, 876–884 (2016).
García-Valverde, R., Cherni, J. A. & Urbina, A. Life cycle analysis of organic photovoltaic technologies. Prog. Photovolt. 18, 535–558 (2010).
Espinosa, N., Hosel, M., Angmo, D. & Krebs, F. C. Solar cells with one-day energy payback for the factories of the future. Energy Environ. Sci. 5, 5117–5132 (2012).
Adams, J. et al. Air-processed organic tandem solar cells on glass: toward competitive operating lifetimes. Energy Environ. Sci. 8, 169–176 (2015).
Acknowledgements
D.B. thanks Helmholtz Association for a Helmholtz Postdoc Fellowship. S.H. thanks BASF for financial support. The authors acknowledge EC FP7 Project SC2 (610115), EC FP7 Project ArtESun (604397), and EPSRC Project EP/G037515/1 and EP/K030671/1, EC FP7 Project POLYMED (612538) and Project Synthetic carbon allotropes project SFB 953.
Author information
Authors and Affiliations
Contributions
D.B. and R.S.A. prepared the manuscript. S.H. and A.W. synthesized the non-fullerene acceptors. R.S.A. fabricated and characterized solar cell devices. D.B. and N.G. carried out CE and TPV measurements. M.A. performed the in situ GIWAXS and UV–Vis absorption measurements during spin coating. D.A.H. performed static GIWAXS measurements. J.A.R. did the SCLC measurements. S.L. performed the DSC measurements. R.S.A. and M.N. performed stability measurements. C.J.M.E. helped J.N. with EROI modelling. All authors discussed the results and commented on the manuscript. C.J.B. supervised charge extraction measurements. J.R.D. supervised TPV measurements, A.A. supervised in situ GIWAXS and UV–Vis measurements, A.S. supervised static GIWAXS measurements, T.K. and J.N. supervised SCLC, electroluminescence and EQE measurements. I.M. revised the manuscript and supervised and directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1058 kb)
Rights and permissions
About this article
Cite this article
Baran, D., Ashraf, R., Hanifi, D. et al. Reducing the efficiency–stability–cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nature Mater 16, 363–369 (2017). https://doi.org/10.1038/nmat4797
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4797
This article is cited by
-
Deuteration-enhanced neutron contrasts to probe amorphous domain sizes in organic photovoltaic bulk heterojunction films
Nature Communications (2024)
-
Rational molecular and device design enables organic solar cells approaching 20% efficiency
Nature Communications (2024)
-
A case study of comparing two dimerized acceptor molecules built by different branch-connected and terminal-connected approaches
Science China Chemistry (2024)
-
A materials physics perspective on structure–processing–function relations in blends of organic semiconductors
Nature Reviews Materials (2023)
-
A dive into underwater solar cells
Nature Photonics (2023)