Abstract
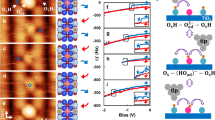
The interaction of water with TiO2 is crucial to many of its practical applications, including photocatalytic water splitting. Following the first demonstration of this phenomenon 40 years ago there have been numerous studies of the rutile single-crystal TiO2(110) interface with water. This has provided an atomic-level understanding of the water–TiO2 interaction. However, nearly all of the previous studies of water/TiO2 interfaces involve water in the vapour phase. Here, we explore the interfacial structure between liquid water and a rutile TiO2(110) surface pre-characterized at the atomic level. Scanning tunnelling microscopy and surface X-ray diffraction are used to determine the structure, which is comprised of an ordered array of hydroxyl molecules with molecular water in the second layer. Static and dynamic density functional theory calculations suggest that a possible mechanism for formation of the hydroxyl overlayer involves the mixed adsorption of O2 and H2O on a partially defected surface. The quantitative structural properties derived here provide a basis with which to explore the atomistic properties and hence mechanisms involved in TiO2 photocatalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Henderson, M. A. A surface science perspective on photocatalysis. Surf. Sci. Rep. 66, 185–297 (2011).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Kurtz, R. L., Stock-Bauer, R., Msdey, T. E., Román, E. & De Segovia, J. L. Synchrotron radiation studies of H2O adsorption on TiO2(110). Surf. Sci. 218, 178–200 (1989).
Henderson, M. A. An HREELS and TPD study of water on TiO2(110): the extent of molecular versus dissociative adsorption. Surf. Sci. 355, 151–166 (1996).
Brookes, I. M., Muryn, C. A. & Thornton, G. Imaging water dissociation on TiO2(110). Phys. Rev. Lett. 87, 266103 (2001).
Bikondoa, O. et al. Direct visualization of defect-mediated dissociation of water on TiO2(110). Nat. Mater. 5, 189–192 (2006).
Wendt, S. et al. Formation and splitting of paired hydroxyl groups on reduced TiO2(110). Phys. Rev. Lett. 96, 066107 (2006).
He, Y. et al. Nucleation and growth of 1D water clusters on rutile TiO2 (011)-2 × 1. J. Phys. Chem. C 113, 10329–10332 (2009).
He, Y., Tilocca, A., Dulub, O., Selloni, A. & Diebold, U. Local ordering and electronic signatures of submonolayer water on anatase TiO2(101). Nat. Mater. 8, 585–589 (2009).
Pang, C. L., Lindsay, R. & Thornton, G. Structure of clean and adsorbate-covered single-crystal rutile TiO2 surfaces. Chem. Rev. 113, 3887–3948 (2013).
Ramamoorthy, M., Vanderbilt, D. & King-Smith, R. D. First-principles calculations of the energetics of stoichiometric TiO2 surfaces. Phys. Rev. B 49, 16721–16727 (1994).
Kristoffersen, H. H. et al. Role of steps in the dissociative adsorption of water on rutile TiO2(110). Phys. Rev. Lett. 110, 146101 (2013).
Zhang, Z. et al. Water as a catalyst: imaging reactions of O2 with partially and fully hydroxylated TiO2(110) surfaces. J. Phys. Chem. C 113, 1908–1916 (2009).
Renner, F. U. et al. Initial corrosion observed on the atomic scale. Nature 439, 707–710 (2006).
Zhang, Z. et al. Ion adsorption at the rutile-water interface: linking molecular and macroscopic properties. Langmuir 20, 4954–4969 (2004).
Ketteler, G. et al. The nature of water nucleation sites on TiO2(110) surfaces revealed by ambient pressure X-ray photoelectron spectroscopy. J. Phys. Chem. C 111, 8278–8282 (2007).
Cheng, J. & Sprik, M. Aligning electronic energy levels at the TiO2/H2O interface. Phys. Rev. B 82, 081406 (2010).
Cheng, J. & Sprik, M. Acidity of the aqueous rutile TiO2(110) surface from density functional theory based molecular dynamics. J. Chem. Theory Comput. 6, 880–889 (2010).
Liu, L.-M., Zhang, C., Thornton, G. & Michaelides, A. Structure and dynamics of liquid water on rutile TiO2(110). Phys. Rev. B 82, 161415 (2010).
Liu, L.-M., Zhang, C., Thornton, G. & Michaelides, A. Reply to “Comment on ‘Structure and dynamics of liquid water on rutile TiO2(110)’”. Phys. Rev. B 85, 167402 (2012).
Raju, M., Kim, S.-Y., van Duin, A. C. T. & Fichthorn, K. A. ReaxFF reactive force field study of the dissociation of water on titania surfaces. J. Phys. Chem. C 117, 10558–10572 (2013).
Zhang, Z. et al. Structure of rutile TiO2(110) in water and 1 molal Rb+ at pH 12: Inter-relationship among surface charge, interfacial hydration structure, and substrate structural displacements. Surf. Sci. 601, 1129–1143 (2007).
Mezhenny, S. et al. STM studies of defect production on the TiO2(110)-(1 × 1) and TiO2(110)-(1 × 2) surfaces induced by UV irradiation. Chem. Phys. Lett. 369, 152–158 (2003).
Maeda, K. Photocatalytic properties of rutile TiO2 powder for overall water splitting. Catal. Sci. Tech. 4, 1949–1953 (2014).
Lindan, P. J. D., Harrison, N. M. & Gillan, M. J. Mixed dissociative and molecular adsorption of water on the rutile (110) surface. Phys. Rev. Lett. 80, 762–765 (1998).
Uetsuka, H., Sasahara, A. & Onishi, H. Topography of the rutile TiO2(110) surface exposed to water and organic solvents. Langmuir 20, 4782–4783 (2004).
Sasahara, A., Kitamura, S., Uetsuka, H. & Onishi, H. Oxygen-atom vacancies imaged by a noncontact atomic force microscope operated in an atmospheric pressure of N2 gas. J. Phys. Chem. B 108, 15735–15737 (2004).
Vitali, L., Ramsey, M. G. & Netzer, F. P. Unusual growth phenomena of group III and group V elements on Si(111) and Ge(111) surfaces. Appl. Surf. Sci. 175–176, 146–156 (2001).
Yim, C. M., Pang, C. L. & Thornton, G. Oxygen vacancy origin of the surface band-gap state of TiO2(110). Phys. Rev. Lett. 104, 036806 (2010).
Wendt, S. et al. The role of interstitial sites in the Ti3d defect state in the band gap of titania. Science 320, 1755–1759 (2008).
Mao, X. et al. Band-gap states of TiO2(110): major contribution from surface defects. J. Phys. Chem. Lett. 4, 3839–3844 (2013).
Cabailh, G. et al. Geometric structure of TiO2(110)(1 × 1): achieving experimental consensus. Phys. Rev. B 75, 241403 (2007).
Duncan, D. A., Allegretti, F. & Woodruff, D. P. Water does partially dissociate on the perfect TiO2(110) surface: a quantitative structure determination. Phys. Rev. B 86, 045411 (2012).
Allegretti, F., O’Brien, S., Polcik, M., Sayago, D. I. & Woodruff, D. P. Adsorption bond length for H2O on TiO2(110): a key parameter for theoretical understanding. Phys. Rev. Lett. 95, 226104 (2005).
Serrano, G. et al. Molecular ordering at the interface between liquid water and rutile TiO2(110). Adv. Mater. Interfaces 2, 1500256 (2015).
Papageorgiou, A. C. et al. Electron traps and their effect on the surface chemistry of TiO2(110). Proc. Natl Acad. Sci. USA 107, 2391–2396 (2010).
Deskins, N. A., Rousseau, R. & Dupuis, M. Defining the role of excess electrons in the surface chemistry of TiO2 . J. Phys. Chem. C 114, 5891–5897 (2010).
Amft, M. et al. A molecular mechanism for the water–hydroxyl balance during wetting of TiO2 . J. Phys. Chem. C 117, 17078–17083 (2013).
Berkelbach, T. C., Lee, H.-S. & Tuckerman, M. E. Concerted hydrogen-bond dynamics in the transport mechanism of the hydrated proton: a first-principles molecular dynamics study. Phys. Rev. Lett. 103, 238302 (2009).
Tocci, G. & Michaelides, A. Solvent-induced proton hopping at a water–oxide interface. J. Phys. Chem. Lett. 5, 474–480 (2014).
Bullard, J. W. & Cima, M. J. Orientation dependence of the isoelectric point of TiO2 (Rutile) surfaces. Langmuir 22, 10264–10271 (2006).
Cheng, H. & Selloni, A. Hydroxide ions at the water/anatase TiO2(101) interface: structure and electronic states from first principles molecular dynamics. Langmuir 26, 11518–11525 (2010).
Cheng, J., Liu, X., Kattirtzi, J. A., VandeVondele, J. & Sprik, M. Aligning electronic and protonic energy levels of proton-coupled electron transfer in water oxidation on aqueous TiO2 . Angew. Chem. Int. Ed. 53, 12046–12050 (2014).
Valdés, A., Qu, Z.-W., Kroes, G.-J., Rossmeisl, J. & Nørskov, J. K. Oxidation and photo-oxidation of water on TiO2 surface. J. Phys. Chem. C 112, 9872–9879 (2008).
Renner, F. U., Gründer, Y. & Zegenhagen, J. Portable chamber for the study of UHV prepared electrochemical interfaces by hard x-ray diffraction. Rev. Sci. Instrum. 78, 033903 (2007).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
VandeVondele, J. et al. Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Acknowledgements
The authors would like to thank M. Nicotra, Y. Zhang and M. Allan for assistance with some measurements. This work was funded by grants from the EPSRC (UK) (EP/C541898/1), M.E.C. (Spain) through project MAT2015-68760-C2-2-P, EU ITN SMALL, EU COST Action CM1104, ERC Advanced Grant (G.Thornton, ENERGYSURF No. 267768), ERC Consolidator Grant (A.M., HeteroIce project No. 616121) and the Royal Society. We are grateful to the London Centre for Nanotechnology and UCL Research Computing for computation resources, and to the UKCP consortium (EP/ F036884/1) for access to Archer.
Author information
Authors and Affiliations
Contributions
G.Thornton, J.Z. and A.M. designed the project. D.S.H., T.W., C.L.P., C.M.Y., D.C.G. and H.H. performed the STM measurements and T.W. analysed the data. T.W. and C.M.Y. performed the UPS experiments with T.W. analysing the data. H.H., G.C., O.B., X.T., R.L. and G.Thornton performed the SXRD measurements and H.H. and X.T. analysed the data. G.Tocci and A.M. conceived, designed and analysed the ab initio calculations. G.Tocci performed the ab initio calculations. H.H., T.W., G.Tocci, C.L.P., A.M. and G.Thornton wrote the manuscript and the Supplementary Information with input from all authors. All authors participated in discussing the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 35713 kb)
Supplementary Information
Supplementary Movie 1 (MOV 22843 kb)
Rights and permissions
About this article
Cite this article
Hussain, H., Tocci, G., Woolcot, T. et al. Structure of a model TiO2 photocatalytic interface. Nature Mater 16, 461–466 (2017). https://doi.org/10.1038/nmat4793
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4793
This article is cited by
-
Unveiling diverse coordination-defined electronic structures of reconstructed anatase TiO2(001)-(1 × 4) surface
Nature Communications (2024)
-
Bubble-water/catalyst triphase interface microenvironment accelerates photocatalytic OER via optimizing semi-hydrophobic OH radical
Nature Communications (2024)
-
Accelerated intestinal wound healing via dual electrostimulation from a soft and biodegradable electronic bandage
Nature Electronics (2024)
-
Enhanced Photocatalytic Activity of Acerola Peel Extract-Coated TiO2 Against Pseudomonas aeruginosa
Journal of Cluster Science (2024)
-
Mechanistic insight on water dissociation on pristine low-index TiO2 surfaces from machine learning molecular dynamics simulations
Nature Communications (2023)