Abstract
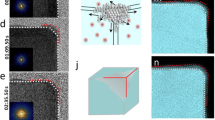
Compositional heterogeneity in shaped, bimetallic nanocrystals offers additional variables to manoeuvre the functionality of the nanocrystal. However, understanding how to manipulate anisotropic elemental distributions in a nanocrystal is a great challenge in reaching higher tiers of nanocatalyst design. Here, we present the evolutionary trajectory of phase segregation in Pt–Ni rhombic dodecahedra. The anisotropic growth of a Pt-rich phase along the 〈111〉 and 〈200〉 directions at the initial growth stage results in Pt segregation to the 14 axes of a rhombic dodecahedron, forming a highly branched, Pt-rich tetradecapod structure embedded in a Ni-rich shell. With longer growth time, the Pt-rich phase selectively migrates outwards through the 14 axes to the 24 edges such that the rhombic dodecahedron becomes a Pt-rich frame enclosing a Ni-rich interior phase. The revealed anisotropic phase segregation and migration mechanism offers a radically different approach to fabrication of nanocatalysts with desired compositional distributions and performance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bell, A. T. The impact of nanoscience on heterogeneous catalysis. Science 299, 1688–1691 (2003).
Debe, M. K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486, 43–51 (2012).
Ferrando, R., Jellinek, J. & Johnston, R. L. Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008).
Narayanan, R. & El-Sayed, M. A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 4, 1343–1348 (2004).
Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. 48, 60–103 (2009).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability. Science 315, 493–497 (2007).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nature Mater. 6, 241–247 (2007).
Wang, C. et al. Design and synthesis of bimetallic electrocatalyst with multilayered Pt-skin surfaces. J. Am. Chem. Soc. 133, 14396–14403 (2011).
Zhang, J., Yang, H., Fang, J. & Zou, S. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 10, 638–644 (2010).
Wu, J. et al. Truncated octahedral Pt3Ni oxygen reduction reaction electrocatalysts. J. Am. Chem. Soc. 132, 4984–4985 (2010).
Cui, C. H. et al. Octahedral PtNi nanoparticle catalysts: exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 12, 5885–5889 (2012).
Cui, C. H., Gan, L., Heggen, M., Rudi, S. & Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nature Mater. 12, 765–771 (2013).
Choi, S.-I. et al. Synthesis and characterization of 9 nm Pt–Ni octahedra with a record high activity of 3.3 A/mgPt for the oxygen reduction reaction. Nano Lett. 13, 3420–3425 (2013).
Huang, X. et al. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Gasteiger, H. A. & Markovic, N. M. Just a dream–or future reality? Science 324, 48–49 (2009).
Gauthier, Y., Joly, Y., Baudoing, R. & Rundgren, J. Surface-sandwich segregation on nondilute bimetallic alloys: Pt50Ni50 and Pt78Ni22 probed by low-energy electron diffraction. Phys. Rev. B 31, 6216–6218 (1985).
Gan, L. Element-specific anisotropic growth of shaped platinum alloy nanocrystals. Science 346, 1502–1506 (2014).
Oh, A. et al. Skeletal octahedral nanoframe with cartesian coordinates via geometrically precise nanoscale phase segregation in a Pt@Ni core–shell nanocrystal. ACS Nano 9, 2856–2867 (2015).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Becknell, N. et al. Atomic structure of Pt3Ni nanoframe electrocatalysts by in situ X-ray absorption spectroscopy. J. Am. Chem. Soc. 137, 15817–15824 (2015).
Becknell, N., Zheng, C., Chen, C., Yu, Y. & Yang, P. Synthesis of PtCo3 polyhedral nanoparticles and evolution to Pt3Co nanoframes. Surf. Sci. 648, 328–332 (2015).
Watt, J., Young, N., Haigh, S., Kirkland, A. & Tilley, R. D. Synthesis and structural characterization of branched palladium nanostructures. Adv. Mater. 21, 2288–2293 (2009).
Wu, Y. et al. Defect-dominated shape recovery of nanocrystals: a new strategy for trimetallic catalysts. J. Am. Chem. Soc. 135, 12220–12223 (2013).
Zeng, J. et al. Controlling the nucleation and growth of silver on palladium nanocubes by manipulating the reaction kinetics. Angew. Chem. Int. Ed. 51, 2354–2358 (2012).
Williams, D. B. & Carter, C. B. The Transmission Electron Microscope (Springer, 1996).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Zabinsky, S., Rehr, J., Ankudinov, A., Albers, R. & Eller, M. FEFF code for ab initio calculations of XAFS. Phys. Rev. B 52, 2995–3009 (1995).
Hwang, B.-J. et al. Structural models and atomic distribution of bimetallic nanoparticles as investigated by X-ray absorption spectroscopy. J. Am. Chem. Soc. 127, 11140–11145 (2005).
Wang, G., Van Hove, M. A., Ross, P. N. & Baskes, M. I. Quantitative prediction of surface segregation in bimetallic Pt–M alloy nanoparticles (M = Ni, Re, Mo). Prog. Surf. Sci. 79, 28–45 (2005).
Wang, G., Van Hove, M. A., Ross, P. N. & Baskes, M. Monte Carlo simulations of segregation in Pt–Ni catalyst nanoparticles. J. Chem. Phys. 122, 024706 (2005).
Ahmadi, M., Behafarid, F., Cui, C. H., Strasser, P. & Cuenya, B. R. Long-range segregation phenomena in shape-selected bimetallic nanoparticles: chemical state effects. ACS Nano 7, 9195–9204 (2013).
Dahmani, C. E., Cadeville, M. C., Sanchez, J. M. & Morán-López, J. L. Ni-Pt phase diagram: experiment and theory. Phys. Rev. Lett. 55, 1208–1211 (1985).
Nash, P. & Singleton, M. The Ni-Pt (nickel-platinum) system. J. Phase Equilib. 10, 258–262 (1989).
Seo, O. et al. Chemical ordering in PtNi nanocrystals. J. Alloys Compd. 666, 232–236 (2016).
Vitos, L., Ruban, A., Skriver, H. L. & Kollar, J. The surface energy of metals. Surf. Sci. 411, 186–202 (1998).
Cui, C. H. et al. Shape-selected bimetallic nanoparticle electrocatalysts: evolution of their atomic-scale structure, chemical composition, and electrochemical reactivity under various chemical environments. Faraday Discuss. 162, 91–112 (2013).
Shao, M., Peles, A. & Shoemaker, K. Electrocatalysis on platinum nanoparticles: particle size effect on oxygen reduction reaction activity. Nano Lett. 11, 3714–3719 (2011).
Xia, Y., Xia, X. & Peng, H.-C. Shape-controlled synthesis of colloidal metal nanocrystals: thermodynamic versus kinetic products. J. Am. Chem. Soc. 137, 7947–7966 (2015).
Zeng, J. et al. Controlling the shapes of silver nanocrystals with different capping agents. J. Am. Chem. Soc. 132, 8552–8553 (2010).
Niu, Z. & Li, Y. Removal and utilization of capping agents in nanocatalysis. Chem. Mater. 26, 72–83 (2013).
Acknowledgements
The research conducted at Lawrence Berkeley National Laboratory was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division, under Contract No. DE-AC02-05CH11231 (surface). Z.N. gratefully acknowledges support from the International Postdoctoral Exchange Fellowship Program 2014. D.K. acknowledges support from Samsung Scholarship. All HRTEM, HAADF-STEM, and EDS mapping made use of the National Center for Electron Microscopy at the Molecular Foundry. XPS data was collected at the Molecular Foundry. We acknowledge M. Marcus and the use of Beamline 10.3.2 at the Advanced Light Source for collection of EXAFS data. The Molecular Foundry and the Advanced Light Source are supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. We acknowledge P. Alivisatos for access to the Bruker D-8 XRD and E. Kreimer of the Microanalytical Facility in the College of Chemistry, UC Berkeley for access to ICP analysis.
Author information
Authors and Affiliations
Contributions
Z.N., N.B. and P.Y. designed the experiments and wrote the paper. Z.N. and N.B. performed the experiments and contributed equally to the work. Y.Y. and N.K. performed HRTEM and HAADF-STEM analysis. D.K. and Z.N. completed XPS analysis. C.C. initiated the work. G.A.S. and P.Y. guided the work. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2656 kb)
Rights and permissions
About this article
Cite this article
Niu, Z., Becknell, N., Yu, Y. et al. Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts. Nature Mater 15, 1188–1194 (2016). https://doi.org/10.1038/nmat4724
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4724
This article is cited by
-
Low-temperature non-equilibrium synthesis of anisotropic multimetallic nanosurface alloys for electrochemical CO2 reduction
Nature Synthesis (2023)
-
Revealing the dynamics of the alloying and segregation of Pt-Co nanoparticles via in-situ environmental transmission electron microscopy
Nano Research (2023)
-
Plasma-enabled synthesis of ordered PtFe alloy nanoparticles encapsulated with ultrathin N-doped carbon shells for efficient methanol electrooxidation
Nano Research (2023)
-
Structure, Property, and Performance of Catalyst Layers in Proton Exchange Membrane Fuel Cells
Electrochemical Energy Reviews (2023)
-
Selective Hydrogenation of Croton Aldehyde on Pt Nanoparticles Controlled by Tailoring Fraction of Well-Ordered Facets Under Different Pretreatment Conditions
Catalysis Letters (2023)