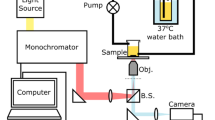
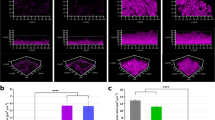
Abstract
Most bacteria in nature exist as biofilms, which support intercellular signalling processes such as quorum sensing (QS), a cell-to-cell communication mechanism that allows bacteria to monitor and respond to cell density and changes in the environment. As QS and biofilms are involved in the ability of bacteria to cause disease, there is a need for the development of methods for the non-invasive analysis of QS in natural bacterial populations. Here, by using surface-enhanced resonance Raman scattering spectroscopy, we report rationally designed nanostructured plasmonic substrates for the in situ, label-free detection of a QS signalling metabolite in growing Pseudomonas aeruginosa biofilms and microcolonies. The in situ, non-invasive plasmonic imaging of QS in biofilms provides a powerful analytical approach for studying intercellular communication on the basis of secreted molecules as signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Waters, C. M. & Bassler, B. L. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 (2005).
Lazdunski, A. M., Ventre, I. & Sturgis, J. N. Regulatory circuits and communication in gram-negative bacteria. Nature Rev. Microbiol. 2, 581–592 (2004).
Rutherford, S. T. & Bassler, B. L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2, a012427 (2012).
LaSarre, B. & Federle, M. J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 77, 73–111 (2013).
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev. Microbiol. 2, 95–108 (2004).
Davey, M. E. & O’toole, G. A. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64, 847–867 (2000).
Li, Y. H. & Tian, X. L. Quorum sensing and bacterial social interactions in biofilms. Sensors 12, 2519–2538 (2012).
Parsek, M. R. & Greenberg, E. P. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13, 27–33 (2005).
Rybtke, M., Hultqvist, L. D., Givskov, M. & Tolker-Nielsen, T. Pseudomonas aeruginosa biofilm infections: community structure, antimicrobial tolerance and immune response. J. Mol. Biol. 427, 3628–3645 (2015).
Jimenez, P. N. et al. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 76, 46–65 (2012).
Castillo-Juarez, I. et al. Role of quorum sensing in bacterial infections. World J. Clin. Cases 3, 575–598 (2015).
Decho, A. W., Norman, R. S. & Visscher, P. T. Quorum sensing in natural environments: emerging views from microbial mats. Trends Microbiol. 18, 73–80 (2010).
Steindler, L. & Venturi, V. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol. Lett. 266, 1–9 (2007).
Jansson, J. K. Marker and reporter genes: illuminating tools for environmental microbiologists. Curr. Opin. Microbiol. 6, 310–316 (2003).
Moskovits, M. Surface-enhanced Raman spectroscopy: a brief retrospective. J. Raman Spectrosc. 36, 485–496 (2005).
Stiles, P. L., Dieringer, J. A., Shah, N. C. & Van Duyne, R. R. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 1, 601–626 (2008).
Schlucker, S. Surface-enhanced Raman spectroscopy: concepts and chemical applications. Angew. Chem. Int. Ed. 53, 4756–4795 (2014).
Anker, J. N. et al. Biosensing with plasmonic nanosensors. Nature Mater. 7, 442–453 (2008).
Bantz, K. C. et al. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 13, 11551–11567 (2011).
Abalde-Cela, S. et al. Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J. R. Soc. Interface 7, S435–S450 (2010).
Alvarez-Puebla, R. A. & Liz-Marzan, L. M. SERS-based diagnosis and biodetection. Small 6, 604–610 (2010).
Howes, P. D., Chandrawati, R. & Stevens, M. M. Colloidal nanoparticles as advanced biological sensors. Science 346, 1247390 (2014).
Zeng, S. W., Baillargeat, D., Ho, H. P. & Yong, K. T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 43, 3426–3452 (2014).
Pierson, L. S. & Pierson, E. A. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 86, 1659–1670 (2010).
Mavrodi, D. V. et al. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 183, 6454–6465 (2001).
Dietrich, L. E. P. et al. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 61, 1308–1321 (2006).
Dietrich, L. E. P., Teal, T. K., Price-Whelan, A. & Newman, D. K. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321, 1203–1206 (2008).
Ramos, I., Dietrich, L. E. P., Price-Whelan, A. & Newman, D. K. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 161, 187–191 (2010).
Dietrich, L. E. P. et al. Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol. 195, 1371–1380 (2013).
Price-Whelan, A., Dietrich, L. E. & Newman, D. K. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nature Chem. Biol. 2, 71–78 (2006).
Lee, J. & Zhang, L. H. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6, 26–41 (2015).
Wu, X. M. et al. Culture-free diagnostics of Pseudomonas aeruginosa infection by silver nanorod array based SERS from clinical sputum samples. Nanomedicine 10, 1863–1870 (2014).
Lopez-Puente, V. et al. Plasmonic mesoporous composites as molecular sieves for SERS detection. J. Phys. Chem. Lett. 4, 2715–2720 (2013).
Yang, S., Dai, X., Boschitsch Stogin, B. & Wong, T. Ultrasensitive surface-enhanced Raman scattering detection in common fluids. Proc. Natl Acad. Sci. USA 113, 268–273 (2015).
Hamon, C. et al. Hierarchical self-assembly of gold nanoparticles into patterned plasmonic nanostructures. ACS Nano 8, 10694–10703 (2014).
Reszka, K. J. et al. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic. Biol. Med. 36, 1448–1459 (2004).
Lebeaux, D., Chauhan, A., Rendueles, O. & Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2, 288–356 (2013).
Coenye, T. & Nelis, H. J. In vitro and in vivo model systems to study microbial biofilm formation. J. Microbiol. Methods 83, 89–105 (2010).
Koley, D., Ramsey, M. M., Bard, A. J. & Whiteley, M. Discovery of a biofilm electrocline using real-time 3D metabolite analysis. Proc. Natl Acad. Sci. USA 108, 19996–20001 (2011).
Bellin, D. L. et al. Integrated circuit-based electrochemical sensor for spatially resolved detection of redox-active metabolites in biofilms. Nature Commun. 5, 3256 (2014).
Pedersen, S. S., Shand, G. H., Hansen, B. L. & Hansen, G. N. Induction of experimental chronic Pseudomonas-aeruginosa lung infection with Pseudomonas-aeruginosa entrapped in alginate microspheres. APMIS 98, 203–211 (1990).
Leevy, W. M., Serazin, N. & Smith, B. D. Optical imaging of bacterial infection models. Drug Discov. Today Dis. Models 4, 91–97 (2007).
Hunter, R. C. et al. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 47, 738–745 (2012).
Reuter, K., Steinbach, A. & Helms, V. Interfering with bacterial quorum sensing. Perspect. Medicin. Chem. 8, 1–15 (2016).
O’Loughlin, C. T. et al. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl Acad. Sci. USA 110, 17981–17986 (2013).
Welsh, M. A., Eibergen, N. R., Moore, J. D. & Blackwell, H. E. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 137, 1510–1519 (2015).
Dai, T. H. et al. Animal models of external traumatic wound infections. Virulence 2, 296–315 (2011).
Wessel, A. K., Hmelo, L., Parsek, M. R. & Whiteley, M. Going local: technologies for exploring bacterial microenvironments. Nature Rev. Microbiol. 11, 337–348 (2013).
Connell, J. L. et al. Real-time monitoring of quorum sensing in 3D-printed bacterial aggregates using scanning electrochemical microscopy. Proc. Natl Acad. Sci. USA 111, 18255–18260 (2014).
Buenger, D., Topuz, F. & Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 37, 1678–1719 (2012).
Weibel, D. B., DiLuzio, W. R. & Whitesides, G. M. Microfabrication meets microbiology. Nature Rev. Microbiol. 5, 209–218 (2007).
Tian, L. et al. Plasmonic biofoam: a versatile optically active material. Nano Lett. 16, 609–616 (2016).
Hamon, C. et al. Hierarchical organization and molecular diffusion in gold nanorod/silica supercrystal nanocomposites. Nanoscale 8, 7914–7922 (2016).
Falconnet, D., Csucs, G., Grandin, H. M. & Textor, M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 27, 3044–3063 (2006).
Shao, Y. & Fu, J. P. Integrated micro/nanoengineered functional biomaterials for cell mechanics and mechanobiology: a materials perspective. Adv. Mater. 26, 1494–1533 (2014).
Mitragotri, S. & Lahann, J. Physical approaches to biomaterial design. Nature Mater. 8, 15–23 (2009).
Watrous, J. D. & Dorrestein, P. C. Imaging mass spectrometry in microbiology. Nature Rev. Microbiol. 9, 683–694 (2011).
Fang, J. S. & Dorrestein, P. C. Emerging mass spectrometry techniques for the direct analysis of microbial colonies. Curr. Opin. Microbiol. 19, 120–129 (2014).
Wang, C., Flynn, N. T. & Langer, R. Controlled structure and properties of thermoresponsive nanoparticle–hydrogel composites. Adv. Mater. 16, 1074–1079 (2004).
Scarabelli, L., Grzelczak, M. & Liz-Marzan, L. M. Tuning gold nanorod synthesis through prereduction with salicylic acid. Chem. Mater. 25, 4232–4238 (2013).
Crepaldi, E. L. et al. Controlled formation of highly organized mesoporous titania thin films: from mesostructured hybrids to mesoporous nanoanatase TiO2 . J. Am. Chem. Soc. 125, 9770–9786 (2003).
Dong Qin, Y. X. & Whitesides, G. M. Soft lithography for micro- and nanoscale patterning. Nature Protoc. 5, 491–502 (2010).
McDonald, J. C. et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21, 27–40 (2000).
Alvarez-Puebla, R. A. et al. Au@pNIPAM colloids as molecular traps for surface-enhanced, spectroscopic, ultra-sensitive analysis. Angew. Chem. Int. Ed. 48, 138–143 (2009).
Kern, S. E. & Newman, D. K. Measurement of phenazines in bacterial cultures. Methods Mol. Biol. 1149, 303–310 (2014).
Acknowledgements
This work has been funded by the European Research Council (ERC Advanced Grant no. 267867 Plasmaquo). We thank D. K. Newman, Department of Biology, California Institute of Technology and Howard Hughes Medical Institute for providing us with P. aeruginosa PA14 and PA14 Δphz1/2 strains. We thank F. M. Ausubel, Department of Genetics, Harvard Medical School for providing P. aeruginosa PA14 phzH, phzM and phzS strains. E. Modin and A. Chuvilin are thanked for their work on FIB/SEM of supercrystals. E.H.H. gratefully acknowledges the Spanish Ministry of Economy and Competitiveness for funding a Juan de la Cierva Fellowship. V.M.-G. acknowledges an FPU scholarship from the Spanish MINECO.
Author information
Authors and Affiliations
Contributions
G.B. designed and executed bacterial growth experiments, V.M.-G. performed SERS experiments with Au@pNIPAM substrates, V.L.-P. fabricated Au@TiO2 substrates and measured SERS, C.C. executed bacterial growth experiments, S.R.-C. and S.C. participated in the fabrication of Au@pNIPAM substrates, I.P.-J. carried out DFT calculations, E.H.H. executed bacterial growth experiments and measured SERS on Au@SiO2 substrates, C.H., M.N.S.-O. and L.S. participated in the fabrication of Au@SiO2 substrates, A.L.P. carried out SEM imaging, I.P.-S. and J.P.-J. contributed to substrate design and discussion, L.M.L.-M. designed and supervised the project and the discussions. All authors participated in writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5821 kb)
Rights and permissions
About this article
Cite this article
Bodelón, G., Montes-García, V., López-Puente, V. et al. Detection and imaging of quorum sensing in Pseudomonas aeruginosa biofilm communities by surface-enhanced resonance Raman scattering. Nature Mater 15, 1203–1211 (2016). https://doi.org/10.1038/nmat4720
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4720
This article is cited by
-
Biofilm formation, occurrence, microbial communication, impact and characterization methods in natural and anthropic systems: a review
Environmental Chemistry Letters (2024)
-
Ultrafast charge transfer in mixed-dimensional WO3-x nanowire/WSe2 heterostructures for attomolar-level molecular sensing
Nature Communications (2023)
-
Chiral molecular imprinting-based SERS detection strategy for absolute enantiomeric discrimination
Nature Communications (2022)
-
Raman microspectroscopy for microbiology
Nature Reviews Methods Primers (2021)
-
Rapid fabrication of complex nanostructures using room-temperature ultrasonic nanoimprinting
Nature Communications (2021)