Abstract
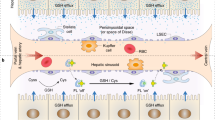
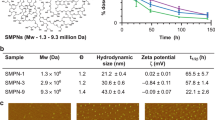
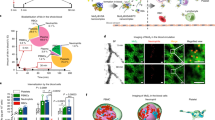
The liver and spleen are major biological barriers to translating nanomedicines because they sequester the majority of administered nanomaterials and prevent delivery to diseased tissue. Here we examined the blood clearance mechanism of administered hard nanomaterials in relation to blood flow dynamics, organ microarchitecture and cellular phenotype. We found that nanomaterial velocity reduces 1,000-fold as they enter and traverse the liver, leading to 7.5 times more nanomaterial interaction with hepatic cells relative to peripheral cells. In the liver, Kupffer cells (84.8 ± 6.4%), hepatic B cells (81.5 ± 9.3%) and liver sinusoidal endothelial cells (64.6 ± 13.7%) interacted with administered PEGylated quantum dots, but splenic macrophages took up less material (25.4 ± 10.1%) due to differences in phenotype. The uptake patterns were similar for two other nanomaterial types and five different surface chemistries. Potential new strategies to overcome off-target nanomaterial accumulation may involve manipulating intra-organ flow dynamics and modulating the cellular phenotype to alter hepatic cell interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kim, B. Y., Rutka, J. T. & Chan, W. C. Nanomedicine. N. Engl. J. Med. 363, 2434–2443 (2010).
Veiseh, O., Tang, B. C., Whitehead, K. A., Anderson, D. G. & Langer, R. Managing diabetes with nanomedicine: challenges and opportunities. Nat. Rev. Drug. Discov. 14, 45–57 (2014).
Mulder, W. J. M., Jaffer, F. A., Fayad, Z. A. & Nahrendorf, M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 6, 239sr1 (2014).
Nasongkla, N. et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 6, 2427–2430 (2006).
Jaiswal, J. K., Mattoussi, H., Mauro, J. M. & Simon, S. M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 21, 47–51 (2002).
Dhar, S., Daniel, W. L., Giljohann, D. A., Mirkin, C. A. & Lippard, S. J. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 131, 14652–14653 (2009).
Kam, N. W. S., O’Connell, M., Wisdom, J. A. & Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl Acad. Sci. USA 102, 11600–11605 (2005).
Wilhelm, S., Tavares, A. J., Dai, Q., Ohta, S. & Audet, J. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Zhang, C. et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. Biomaterials 29, 1233–1241 (2008).
Fonge, H., Huang, H., Scollard, D., Reilly, R. M. & Allen, C. Influence of formulation variables on the biodistribution of multifunctional block copolymer micelles. J. Control. Release 157, 366–374 (2012).
Ye, L. et al. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nat. Nanotech. 7, 453–458 (2012).
Fischer, H. C., Liu, L., Pang, K. S. & Chan, W. C. W. Pharmacokinetics of nanoscale quantum dots: in vivo distribution, sequestration, and clearance in the rat. Adv. Funct. Mater. 16, 1299–1305 (2006).
Semmler-Behnke, M. et al. Biodistribution of 1.4- and 18-nm gold particles in rats. Small 4, 2108–2111 (2008).
De Jong, W. H. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29, 1912–1919 (2008).
Liu, Z. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotech. 2, 47–52 (2006).
Yang, S. T. et al. Biodistribution of pristine single-walled carbon nanotubes in vivo. J. Phys. Chem. C 111, 17761–17764 (2007).
Hauck, T. S., Anderson, R. E., Fischer, H. C., Newbigging, S. & Chan, W. C. W. In vivo quantum-dot toxicity assessment. Small 6, 138–144 (2010).
Kuwahira, I., Gonzalez, N. C., Heisler, N. & Piiper, J. Changes in regional blood flow distribution and oxygen supply during hypoxia in conscious rats. J. Appl. Physiol. 74, 211–214 (1993).
Fournier, L. S. et al. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur. Radiol. 14, 2125–2133 (2004).
Miyazaki, S. et al. Investigation on the optimal position for the quantification of hepatic perfusion by use of dynamic contrast-enhanced computed tomography in rats. Radiol. Phys. Technol. 2, 183–188 (2009).
Menger, M. D., Marzi, I. & Messmer, K. In vivo fluorescence microscopy for quantitative analysis of the hepatic microcirculation in hamsters and rats. Eur. Surg. Res. 23, 158–169 (1991).
MacPhee, P. J., Schmidt, E. E. & Groom, A. C. Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. Am. J. Physiol. Gastrointest. Liver Physiol. 269, G692–G698 (1995).
Ingham, D. B. Diffusion of aerosols from a stream flowing through a cylindrical tube. J. Aerosol. Sci. 6, 125–132 (1975).
Davies, C. N. Diffusion and sedimentation of aerosol particles from Poiseuille flow in pipes. J. Aerosol. Sci. 4, 317–328 (1973).
Dobrovolskaia, M. A. & McNeil, S. E. Immunological properties of engineered nanomaterials. Nat. Nanotech. 2, 469–478 (2007).
Lunov, O. et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 5, 1657–1669 (2011).
Wang, H., Wu, L. & Reinhard, B. M. Scavenger receptor mediated endocytosis of silver nanoparticles into J774A.1 macrophages is heterogeneous. ACS Nano 6, 7122–7132 (2012).
Sykes, E. A., Chen, J., Zheng, G. & Chan, W. C. W. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 8, 5696–5706 (2014).
Perrault, S. D., Walkey, C., Jennings, T., Fischer, H. C. & Chan, W. C. W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9, 1909–1915 (2009).
Cho, W.-S. et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 245, 116–123 (2010).
Chithrani, B. D. & Chan, W. C. W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7, 1542–1550 (2007).
Gao, H. J., Shi, W. D. & Freund, L. B. Mechanics of receptor-mediated endocytosis. Proc. Natl Acad. Sci. USA 102, 9469–9474 (2005).
Cormode, D. P. et al. A versatile and tunable coating strategy allows control of nanocrystal delivery to cell types in the liver. Bioconjug. Chem. 22, 353–361 (2011).
Cheng, S.-H. et al. Visualizing dynamics of sub-hepatic distribution of nanoparticles using intravital multiphoton fluorescence microscopy. ACS Nano 6, 4122–4131 (2012).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).
Sadauskas, E. et al. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 4, 10–17 (2007).
Bartneck, M. et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano 6, 8767–8777 (2012).
Nakashima, M. et al. Pivotal advance: characterization of mouse liver phagocytic B cells in innate immunity. J. Leukoc. Biol. 91, 537–546 (2012).
Stock, R. J., Cilento, E. V., Reilly, F. D. & McCuskey, R. S. A compartmental analysis of the splenic circulation in rat. Am. J. Physiol. Heart Circ. Physiol. 245, H17–H21 (1983).
Chadburn, A. The spleen: anatomy and anatomical function. Semin. Hematol. 37, 13–21 (2000).
Delp, M. D., Evans, M. V. & Duan, C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J. Appl. Physiol. 85, 1813–1822 (1998).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Jones, S. W. et al. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J. Clin. Invest. 123, 3061–3073 (2013).
Koo, A., Liang, I. Y. & Cheng, K. K. Hepatic sinusoidal responses to intraportal injections of phenylephrine and isoprenaline in the rat. Clin. Exp. Pharmacol. Physiol. 3, 391–395 (1976).
Hagemann, T. et al. ‘Re-educating’ tumor-associated macrophages by targeting NF-B. J. Exp. Med. 205, 1261–1268 (2008).
Acknowledgements
We would to acknowledge the Canadian Institute of Health Research, Natural Sciences and Engineering Research Council, and Collaborative Health Research Program for funding the project. K.M.T. thanks the NSERC Vanier Canada Graduate Scholarship Program and the Surgeon-Scientist Program at the University of Toronto for financial support. S.A.M. thanks the CASL/CIHR Hepatology Fellowship Program and the National CIHR Research Training Program in Hepatitis C for financial support. We would also like to acknowledge M. Peralta and C. Hoculada from the University Health Network Pathology Research Program (Toronto, Canada), D. Holmyard from the Mount Sinai Advanced Bioimaging Centre (Toronto, Canada), F. Xu from the University Health Network Advanced Optical Microscopy Facility (Toronto, Canada), D. White from the Department of Immunology, University of Toronto (Toronto, Ontario), J. Manual, J. Feld and V. Cherepanov from the Toronto Centre for Liver Disease (Toronto, Canada), J. Krieger from the SPARC Biocentre at the Hospital for Sick Children (Toronto, Canada) and A. Black from the Department of Anatomy, National University of Ireland, Galway (Galway, Ireland) for their assistance.
Author information
Authors and Affiliations
Contributions
K.M.T., S.A.M., J.B.C., I.D.M. and W.C.W.C. conceived the idea. K.M.T., S.A.M., O.A.A., I.D.M. and W.C.W.C. analysed the data. K.M.T. and S.A.M. conducted the experiments with assistance from X.-Z.M., V.N.S., J.E., B.O., S.M.F., E.A.S., N.G. and J.M.K. A.Z. performed the mathematical modelling. B.A.A., M.S. and M.A.O. supervised some of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 8315 kb)
Supplementary Movie 1
Supplementary Movie 1 (MP4 2858 kb)
Supplementary Movie 2
Supplementary Movie 2 (MP4 2499 kb)
Supplementary Information
Characterization of the protein corona (XLSX 46 kb)
Supplementary Information
Mathematica code (PDF 478 kb)
Rights and permissions
About this article
Cite this article
Tsoi, K., MacParland, S., Ma, XZ. et al. Mechanism of hard-nanomaterial clearance by the liver. Nature Mater 15, 1212–1221 (2016). https://doi.org/10.1038/nmat4718
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4718