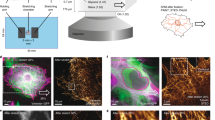
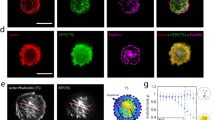
Abstract
Mechanical homeostasis—a fundamental process by which cells maintain stable states under environmental perturbations—is regulated by two subcellular mechanotransducers: cytoskeleton tension and integrin-mediated focal adhesions (FAs)1,2,3,4,5. Here, we show that single-cell mechanical homeostasis is collectively driven by the distinct, graduated dynamics (rheostasis) of subcellular cytoskeleton tension and FAs. Such rheostasis involves a mechanosensitive pattern wherein ground states of cytoskeleton tension and FA determine their distinct reactive paths through either relaxation or reinforcement. Pharmacological perturbations of the cytoskeleton and molecularly modulated integrin catch–slip bonds biased the rheostasis and induced non-homeostasis of FAs, but not of cytoskeleton tension, suggesting a unique sensitivity of FAs in regulating homeostasis. Theoretical modelling revealed myosin-mediated cytoskeleton contractility and catch–slip-bond-like behaviours in FAs and the cytoskeleton as sufficient and necessary mechanisms for quantitatively recapitulating mechanosensitive rheostasis. Our findings highlight the previously underappreciated physical nature of the mechanical homeostasis of cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wang, N. et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 282, C606–C616 (2002).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Solon, J., Levental, I., Sengupta, K., Georges, P. C. & Janmey, P. A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93, 4453–4461 (2007).
Rape, A. D., Guo, W.-H. & Wang, Y.-L. The regulation of traction force in relation to cell shape and focal adhesions. Biomaterials 32, 2043–2051 (2011).
Webster, K. D., Ng, W. P. & Fletcher, D. A. Tensional homeostasis in single fibroblasts. Biophys. J. 107, 146–155 (2014).
Schwartz, M. W. et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66 (2013).
Ritsma, L. et al. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507, 362–365 (2014).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011).
Vermeulen, L. & Snippert, H. J. Stem cell dynamics in homeostasis and cancer of the intestine. Nature Rev. Cancer 14, 468–480 (2014).
Chen, C. S. Mechanotransduction—a field pulling together? J. Cell Sci. 121, 3285–3292 (2008).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Rev. Mol. Cell Biol. 7, 265–275 (2006).
Deakin, N. O. & Turner, C. E. Paxillin comes of age. J. Cell Sci. 121, 2435–2444 (2008).
Galbraith, C. G., Yamada, K. M. & Sheetz, M. P. The relationship between force and focal complex development. J. Cell Biol. 159, 695–705 (2002).
Oakes, P. W., Beckham, Y., Stricker, J. & Gardel, M. L. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 196, 363–374 (2012).
Chen, Y., Pasapera, A. M., Koretsky, A. P. & Waterman, C. M. Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc. Natl Acad. Sci. USA 110, E2352–E2361 (2013).
Ezratty, E. J., Partridge, M. A. & Gundersen, G. G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nature Cell Biol. 7, 581–590 (2005).
Allingham, J. S., Smith, R. & Rayment, I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nature Struct. Mol. Biol. 12, 378–379 (2005).
Holzinger, A. Jasplakinolide: an actin-specific reagent that promotes actin polymerization. Methods Mol. Biol. 586, 71–87 (2009).
Flanagan, M. D. & Lin, S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J. Biol. Chem. 255, 835–838 (1980).
Guo, B. & Guilford, W. H. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc. Natl Acad. Sci. USA 103, 9844–9849 (2006).
Ferrer, J. M. et al. Measuring molecular rupture forces between single actin filaments and actin-binding proteins. Proc. Natl Acad. Sci. USA 105, 9221–9226 (2008).
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009).
Kong, F., García, A. J., Mould, A. P., Humphries, M. J. & Zhu, C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 185, 1275–1284 (2009).
Luo, T. et al. Understanding the cooperative interaction between myosin II and actin cross-linkers mediated by actin filaments during mechanosensation. Biophys. J. 102, 238–247 (2012).
Lee, C.-Y. et al. Actin depolymerization under force is governed by lysine 113:glutamic acid 195-mediated catch-slip bonds. Proc. Natl Acad. Sci. USA 110, 5022–5027 (2013).
Luo, T., Mohan, K., Iglesias, P. A. & Robinson, D. N. Molecular mechanisms of cellular mechanosensing. Nature Mater. 12, 1064–1071 (2013).
Mitrossilis, D. et al. Single-cell response to stiffness exhibits muscle-like behavior. Proc. Natl Acad. Sci. USA 106, 18243–18248 (2009).
Chen, C. et al. Fluidization and resolidification of the human bladder smooth muscle cell in response to transient stretch. PLoS ONE 5, e12035 (2010).
Tondon, A., Hsu, H.-J. & Kaunas, R. Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J. Biomech. 45, 728–735 (2012).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Veigel, C., Molloy, J. E., Schmitz, S. & Kendrick-Jones, J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nature Cell Biol. 5, 980–986 (2003).
Kumar, S. et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys. J. 90, 3762–3773 (2006).
Lu, L., Oswald, S. J., Ngu, H. & Yin, F. C. P. Mechanical properties of actin stress fibers in living cells. Biophys. J. 95, 6060–6071 (2008).
Mann, J. M., Lam, R. H. W., Weng, S., Sun, Y. & Fu, J. A silicone-based stretchable micropost array membrane for monitoring live-cell subcellular cytoskeletal response. Lab Chip 12, 731–740 (2012).
du Roure, O. Force mapping in epithelial cell migration. Proc. Natl Acad. Sci. USA 102, 2390–2395 (2005).
Saez, A., Buguin, A., Silberzan, P. & Ladoux, B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J. 89, L52–L54 (2005).
Fu, J. et al. Mechanical regulation of cell function using geometrically modulated elastomeric substrates. Nature Methods 7, 733–736 (2010).
Yang, M. T., Fu, J., Wang, Y.-K., Desai, R. A. & Chen, C. S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nature Protoc. 6, 187–213 (2011).
Alexandrova, A. Y. et al. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 3, e3234 (2008).
Li, Y. et al. Age-associated increase of skin fibroblast-derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin. J. Invest. Dermatol. 135, 2181–2188 (2015).
Lu, S. et al. Decipher the dynamic coordination between enzymatic activity and structural modulation at focal adhesions in living cells. Sci. Rep. 4, 5756 (2014).
Acknowledgements
We thank A. Bershadsky for providing REF-52 cells, G. J. Fisher for providing human skin fibroblasts, and A. Liu for comments on the manuscript. This work is supported by the National Science Foundation (CMMI 1129611 and CBET 1149401), the National Institutes of Health (R21 HL114011 and R21 EB017078), the American Heart Association (12SDG12180025), and the Department of Mechanical Engineering at the University of Michigan, Ann Arbor. The Lurie Nanofabrication Facility at the University of Michigan, a member of the National Nanotechnology Infrastructure Network (NNIN) funded by the National Science Foundation, is acknowledged for support in microfabrication.
Author information
Authors and Affiliations
Contributions
S.W. and J.F. designed experiments; S.W., Y.S. and W.C. performed experiments and modelling; S.W., Y.S. and J.F. analysed data and wrote the manuscript; J.F. supervised the project. All authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 14497 kb)
Rights and permissions
About this article
Cite this article
Weng, S., Shao, Y., Chen, W. et al. Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis. Nature Mater 15, 961–967 (2016). https://doi.org/10.1038/nmat4654
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4654
This article is cited by
-
The spatiotemporal heterogeneity of the biophysical microenvironment during hematopoietic stem cell development: from embryo to adult
Stem Cell Research & Therapy (2023)
-
What are the key mechanical mechanisms governing integrin-mediated cell migration in three-dimensional fiber networks?
Biomechanics and Modeling in Mechanobiology (2023)
-
Microskeletal stiffness promotes aortic aneurysm by sustaining pathological vascular smooth muscle cell mechanosensation via Piezo1
Nature Communications (2022)
-
Biomechanical cues as master regulators of hematopoietic stem cell fate
Cellular and Molecular Life Sciences (2021)
-
Mechanical homeostasis in tissue equivalents: a review
Biomechanics and Modeling in Mechanobiology (2021)