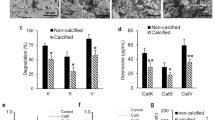
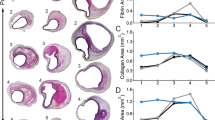
Abstract
Clinical evidence links arterial calcification and cardiovascular risk. Finite-element modelling of the stress distribution within atherosclerotic plaques has suggested that subcellular microcalcifications in the fibrous cap may promote material failure of the plaque, but that large calcifications can stabilize it. Yet the physicochemical mechanisms underlying such mineral formation and growth in atheromata remain unknown. Here, by using three-dimensional collagen hydrogels that mimic structural features of the atherosclerotic fibrous cap, and high-resolution microscopic and spectroscopic analyses of both the hydrogels and of calcified human plaques, we demonstrate that calcific mineral formation and maturation results from a series of events involving the aggregation of calcifying extracellular vesicles, and the formation of microcalcifications and ultimately large calcification areas. We also show that calcification morphology and the plaque’s collagen content—two determinants of atherosclerotic plaque stability—are interlinked.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mozaffarian, D. et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–322 (2015).
Murray, C. J. & Lopez, A. D. Measuring the global burden of disease. N. Engl. J. Med. 369, 448–457 (2013).
Libby, P. Collagenases and cracks in the plaque. J. Clin. Invest. 123, 3201–3203 (2013).
Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 368, 2004–2013 (2013).
Kelly-Arnold, A. et al. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc. Natl Acad. Sci. USA 110, 10741–10746 (2013).
Maldonado, N., Kelly-Arnold, A., Cardoso, L. & Weinbaum, S. The explosive growth of small voids in vulnerable cap rupture; cavitation and interfacial debonding. J. Biomech. 46, 396–401 (2013).
Maldonado, N. et al. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: potential implications for plaque rupture. Am. J. Physiol. Heart. Circ. Physiol. 303, H619–628 (2012).
Vliegenthart, R. et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 112, 572–577 (2005).
Martin, S. S. et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 129, 77–86 (2014).
Vengrenyuk, Y. et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl Acad. Sci. USA 103, 14678–14683 (2006).
Criqui, M. H. et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. J. Am. Med. Assoc. 311, 271–278 (2014).
Lin, T. C. et al. Mechanical response of a calcified plaque model to fluid shear force. Ann. Biomed. Eng. 34, 1535–1541 (2006).
Imoto, K. et al. Longitudinal structural determinants of atherosclerotic plaque vulnerability: a computational analysis of stress distribution using vessel models and three-dimensional intravascular ultrasound imaging. J. Am. Coll. Cardiol. 46, 1507–1515 (2005).
Wong, K. K., Thavornpattanapong, P., Cheung, S. C., Sun, Z. & Tu, J. Effect of calcification on the mechanical stability of plaque based on a three-dimensional carotid bifurcation model. BMC Cardiovasc. Disord. 12, 7 (2012).
Holzapfel, G. A., Mulvihill, J. J., Cunnane, E. M. & Walsh, M. T. Computational approaches for analyzing the mechanics of atherosclerotic plaques: a review. J. Biomech. 47, 859–869 (2014).
Ehara, S. et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 110, 3424–3429 (2004).
Bertazzo, S. et al. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nature Mater. 12, 576–583 (2013).
New, S. E. et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 113, 72–77 (2013).
Kapustin, A. N. et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 109, e1–12 (2011).
Anderson, H. C. Matrix vesicles and calcification. Curr. Rheum. Rep. 5, 222–226 (2003).
Maldonado, N., Kelly-Arnold, A., Laudier, D., Weinbaum, S. & Cardoso, L. Imaging and analysis of microcalcifications and lipid/necrotic core calcification in fibrous cap atheroma. Int. J. Cardiovasc. Image 31, 1079–1087 (2015).
Krahn, K. N., Bouten, C. V., van Tuijl, S., van Zandvoort, M. A. & Merkx, M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal. Biochem. 350, 177–185 (2006).
Hutcheson, J. D. et al. Enrichment of calcifying extracellular vesicles using density-based ultracentrifugation protocol. J. Extracell. Ves. 3, 25129 (2014).
Pleshko, N., Boskey, A. & Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 60, 786–793 (1991).
Gadaleta, S. J., Paschalis, E. P., Betts, F., Mendelsohn, R. & Boskey, A. L. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: new correlations between X-ray diffraction and infrared data. Calc. Tissue Int. 58, 9–16 (1996).
Thouverey, C. et al. Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J. Proteomic. 74, 1123–1134 (2011).
Xiao, Z. et al. Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J. Cell. Physiol. 210, 325–335 (2007).
Walsh, B. J., Thornton, S. C., Penny, R. & Breit, S. N. Microplate reader-based quantitation of collagens. Anal. Biochem. 203, 187–190 (1992).
Aikawa, E. et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 116, 2841–2850 (2007).
Aikawa, E. et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation 119, 1785–1794 (2009).
Aikawa, E. et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115, 377–386 (2007).
Goettsch, C. et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Invest. 123, 4731–4738 (2013).
Quillard, T., Araujo, H. A., Franck, G., Tesmenitsky, Y. & Libby, P. Matrix metalloproteinase-13 predominates over matrix metalloproteinase-8 as the functional interstitial collagenase in mouse atheromata. Arterioscler. Thromb. Vasc. Biol. 34, 1179–1186 (2014).
Fraley, R., Wilschut, J., Duzgunes, N., Smith, C. & Papahadjopoulos, D. Studies on the mechanism of membrane fusion: role of phosphate in promoting calcium ion induced fusion of phospholipid vesicles. Biochemistry 19, 6021–6029 (1980).
Wilschut, J., Duzgunes, N., Fraley, R. & Papahadjopoulos, D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry 19, 6011–6021 (1980).
Deguchi, J. O. et al. Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation 112, 2708–2715 (2005).
Nishimura, R. et al. Osterix regulates calcification and degradation of chondrogenic matrices through matrix metalloproteinase 13 (MMP13) expression in association with transcription factor Runx2 during endochondral ossification. J. Biol. Chem. 287, 33179–33190 (2012).
Cardoso, L., Kelly-Arnold, A., Maldonado, N., Laudier, D. & Weinbaum, S. Effect of tissue properties, shape and orientation of microcalcifications on vulnerable cap stability using different hyperelastic constitutive models. J. Biomech. 47, 870–877 (2014).
Ruiz, J. L., Hutcheson, J. D. & Aikawa, E. Cardiovascular calcification: current controversies and novel concepts. Cardiovasc. Pathol. 24, 207–212 (2015).
Goettsch, C. et al. Nuclear factor of activated T cells mediates oxidised LDL-induced calcification of vascular smooth muscle cells. Diabetologia 54, 2690–2701 (2011).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nature Mater. 13, 645–652 (2014).
Adamson, P. D., Vesey, A. T., Joshi, N. V., Newby, D. E. & Dweck, M. R. Salt in the wound: (18)F-fluoride positron emission tomography for identification of vulnerable coronary plaques. Cardiovasc. Diagn. Ther. 5, 150–155 (2015).
Whittaker, P., Kloner, R. A., Boughner, D. R. & Pickering, J. G. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 89, 397–410 (1994).
Yabusaki, K. et al. A novel quantitative approach for eliminating sample-to-sample variation using a hue saturation value analysis program. PLoS ONE 9, e89627 (2014).
Steitz, S. A. et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 89, 1147–1154 (2001).
Filipe, V., Hawe, A. & Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharmaceut. Res. 27, 796–810 (2010).
Wright, M. Nanoparticle tracking analysis for the multiparameter characterization and counting of nanoparticle suspensions. Methods Mol. Biol. 906, 511–524 (2012).
Staudt, T., Lang, M. C., Medda, R., Engelhardt, J. & Hell, S. W. 2,2′-thiodiethanol: a new water soluble mounting medium for high resolution optical microscopy. Microsc. Res. Tech. 70, 1–9 (2007).
Langhorst, M. F., Schaffer, J. & Goetze, B. Structure brings clarity: structured illumination microscopy in cell biology. Biotechnol. J. 4, 858–865 (2009).
Acknowledgements
This work was supported by a research grant from Kowa Company to M.A. E.A. is supported by grants from the National Institutes of Health (R01HL114805; R01HL109506) and a Harvard Catalyst Advanced Microscopy Pilot Award. Harvard Catalyst support is provided by The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centres. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centres, or the National Institutes of Health. P.L. is supported by R01HL80472. The authors thank T. Pham, J. Choi and B. Pieper for technical assistance and C. Swallom for editorial expertise. T. Holmes and M. Siciliano from the Brigham and Women’s Hospital Anatomic Pathology Laboratory graciously provided human autopsy tissue. G. Sukhova provided surgically resected carotid artery tissue from endarterectomy patients at Brigham and Women’s Hospital. S. Singh contributed valuable insight into data interpretation and presentation. Two-photon microscopy and structured illumination imaging were performed at the Harvard Center for Biological Imaging. S.B. was supported by the Junior Research Fellowship scheme from Imperial College London.
Author information
Authors and Affiliations
Contributions
J.D.H. designed the study, performed sample preparation, conducted experimental work, carried out data interpretation and drafted the manuscript. C.G. aided with study design and data interpretation. S.B. performed DDC-SEM and EDS experiments. N.M. performed microCT analysis. J.L.R. aided with super-resolution microscopy and FTIR spectroscopy. W.G. aided with analysis of FTIR spectroscopy. K.Y. performed image segmentation analysis of histological images. T.F. performed histological tissue assessment. C.B. provided a custom collagen probe. G.F. and T.Q. helped obtain and analyse tissue from the MMP-13-deficient mice. P.L. directed the generation of the MMP-13-deficient mice and provided the human carotid specimens. C.B., M.A., P.L. and S.W. provided critical review of the manuscript. E.A. provided the study concept, participated in study design and data interpretation, and aided in manuscript development.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 844 kb)
Rights and permissions
About this article
Cite this article
Hutcheson, J., Goettsch, C., Bertazzo, S. et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nature Mater 15, 335–343 (2016). https://doi.org/10.1038/nmat4519
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4519
This article is cited by
-
Altered Caveolin-1 Dynamics Result in Divergent Mineralization Responses in Bone and Vascular Calcification
Cellular and Molecular Bioengineering (2023)
-
Intracranial Spotty Calcium Predicts Recurrent Stroke in Patients with Symptomatic Intracranial Atherosclerotic Stenosis
Clinical Neuroradiology (2023)
-
Coronary calcium density in relation to coronary heart disease and cardiovascular disease in adults with diabetes or metabolic syndrome: the Multi-ethnic Study of Atherosclerosis (MESA)
BMC Cardiovascular Disorders (2022)
-
Programmed cell death in atherosclerosis and vascular calcification
Cell Death & Disease (2022)
-
Activation of neutral sphingomyelinase 2 through hyperglycemia contributes to endothelial apoptosis via vesicle-bound intercellular transfer of ceramides
Cellular and Molecular Life Sciences (2022)