Abstract
The microscopic kinetics of ubiquitous solid–solid phase transitions remain poorly understood. Here, by using single-particle-resolution video microscopy of colloidal films of diameter-tunable microspheres, we show that transitions between square and triangular lattices occur via a two-step diffusive nucleation pathway involving liquid nuclei. The nucleation pathway is favoured over the direct one-step nucleation because the energy of the solid/liquid interface is lower than that between solid phases. We also observed that nucleation precursors are particle-swapping loops rather than newly generated structural defects, and that coherent and incoherent facets of the evolving nuclei exhibit different energies and growth rates that can markedly alter the nucleation kinetics. Our findings suggest that an intermediate liquid should exist in the nucleation processes of solid–solid transitions of most metals and alloys, and provide guidance for better control of the kinetics of the transition and for future refinements of solid–solid transition theory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

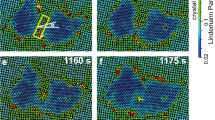
 two-step nucleation in a crystal with two vacancies in an H = 3 μm sample at 27.2 °C.
two-step nucleation in a crystal with two vacancies in an H = 3 μm sample at 27.2 °C.

 two-step nucleation in a crystal with dislocations at 27.4 °C.
two-step nucleation in a crystal with dislocations at 27.4 °C.

 two-step nucleation at a grain boundary at 27.2 °C.
two-step nucleation at a grain boundary at 27.2 °C.


Similar content being viewed by others
References
Porter, D. A., Easterling, K. E. & Sherif, M. Y. Phase Transformations in Metals and Alloys (CRC Press, 2008).
Kirby, S. H., Durham, W. B. & Stern, L. A. Mantle phase changes and deep-earthquake faulting in subducting lithosphere. Science 252, 216–225 (1991).
Irifune, T., Kurio, A., Sakamoto, S., Inoue, T. & Sumiya, H. Ultrahard polycrystalline diamond from graphite. Nature 421, 599–600 (2003).
Smith, W. F. Principles of Materials Science and Engineering (McGraw-Hill, 1996).
Erskine, D. J. & Nellis, W. J. Shock-induced martensitic phase transformation of oriented graphite to diamond. Nature 349, 317–319 (1991).
Khaliullin, R. Z., Eshet, H., Kühne, T. D., Behler, J. & Parrinello, M. Nucleation mechanism for the direct graphite-to-diamond phase transition. Nature Mater. 10, 693–697 (2011).
Burnley, P. C. & Green, H. W. Stress dependence of the mechanism of the olivine–spinel transformation. Nature 338, 753–756 (1989).
Jacobs, K., Zaziski, D., Scher, E. C., Herhold, A. B. & Alivisatos, A. P. Activation volumes for solid–solid transformations in nanocrystals. Science 293, 1803–1806 (2001).
Scandolo, S., Bernasconi, M., Chiarotti, G., Focher, P. & Tosatti, E. Pressure-induced transformation path of graphite to diamond. Phys. Rev. Lett. 74, 4015–4018 (1995).
Zipoli, F., Bernasconi, M. & Martoňák, R. Constant pressure reactive molecular dynamics simulations of phase transitions under pressure: The graphite to diamond conversion revisited. Eur. Phys. J. B 39, 41–47 (2004).
Zahn, D. & Leoni, S. Nucleation and growth in pressure-induced phase transitions from molecular dynamics simulations: Mechanism of the reconstructive transformation of NaCl to the CsCl-type structure. Phys. Rev. Lett. 92, 250201 (2004).
Mundy, C. J. et al. Ultrafast transformation of graphite to diamond: An ab initio study of graphite under shock compression. J. Chem. Phys. 128, 184701 (2008).
Toledano, P. & Dmitriew, V. Reconstructive Phase Transitions in Crystals and Quasicrystals (World Scientific, 1996).
Anderson, V. J. & Lekkerkerker, H. N. W. Insights into phase transition kinetics from colloid science. Nature 416, 811–815 (2002).
Gasser, U., Weeks, E. R., Schofield, A., Pusey, P. N. & Weitz, D. A. Real-space imaging of nucleation and growth in colloidal crystallization. Science 292, 258–262 (2001).
Tan, P., Xu, N. & Xu, L. Visualizing kinetic pathways of homogeneous nucleation in colloidal crystallization. Nature Phys. 10, 73–79 (2014).
Alsayed, A. M., Islam, M. F., Zhang, J., Collings, P. J. & Yodh, A. G. Premelting at defects within bulk colloidal crystals. Science 309, 1207–1210 (2005).
Wang, Z., Wang, F., Peng, Y., Zheng, Z. & Han, Y. Imaging the homogeneous nucleation during the melting of superheated colloidal crystals. Science 338, 87–90 (2012).
Savage, J. R., Blair, D. W., Levine, A. J., Guyer, R. A. & Dinsmore, A. D. Imaging the sublimation dynamics of colloidal crystallites. Science 314, 795–798 (2006).
Weeks, E. R., Crocker, J. C., Levitt, A. C., Schofield, A. & Weitz, D. A. Three-dimensional direct imaging of structural relaxation near the colloidal glass transition. Science 287, 627–631 (2000).
Zhang, Z. et al. Thermal vestige of the zero-temperature jamming transition. Nature 459, 230–233 (2009).
Yethiraj, A., Wouterse, A., Groh, B. & van Blaaderen, A. Nature of an electric-field-induced colloidal martensitic transition. Phys. Rev. Lett. 92, 058301 (2004).
Weiss, J. A., Oxtoby, D. W., Grier, D. G. & Murray, C. A. Martensitic transition in a confined colloidal suspension. J. Chem. Phys. 103, 1180–1190 (1995).
Bolhuis, P. & Frenkel, D. Prediction of an expanded-to-condensed transition in colloidal crystals. Phys. Rev. Lett. 72, 2211–2214 (1994).
Casey, M. T. et al. Driving diffusionless transformations in colloidal crystals using DNA handshaking. Nature Commun. 3, 1209 (2012).
Pieranski, P., Strzelecki, L. & Pansu, B. Thin colloidal crystals. Phys. Rev. Lett. 50, 900–903 (1983).
Schmidt, M. & Löwen, H. Freezing between two and three dimensions. Phys. Rev. Lett. 76, 4552–4555 (1996).
Fortini, A. & Dijkstra, M. Phase behaviour of hard spheres confined between parallel hard plates. J. Phys. Condens. Matter 18, L371–L378 (2006).
Mitchell, T. B. et al. Direct observations of structural phase transitions in planar crystallized ion plasmas. Science 282, 1290–1293 (1998).
Narasimhan, S. & Ho, T-L. Wigner-crystal phases in bilayer quantum Hall systems. Phys. Rev. B 52, 12291–12306 (1995).
Crocker, J. C. & Grier, D. G. Methods of digital video microscopy for colloidal studies. J. Colloid Interf. Sci. 179, 298–310 (1996).
Ten Wolde, P. R. & Frenkel, D. Enhancement of protein crystal nucleation by critical density fluctuations. Science 277, 1975–1978 (1997).
Shekar, N. C. & Rajan, K. G. Kinetics of pressure induced structural phase transitions. Bull. Mater. Sci. 24, 1–21 (2001).
Bai, X-M. & Li, M. Ring-diffusion mediated homogeneous melting in the superheating regime. Phys. Rev. B 77, 134109 (2008).
Levitas, V. I., Henson, B. F., Smilowitz, L. B. & Asay, B. W. Solid–solid phase transformation via virtual melting significantly below the melting temperature. Phys. Rev. Lett. 92, 235702 (2004).
Auer, S. & Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 409, 1020–1023 (2001).
Lundrigan, S. E. & Saika-Voivod, I. Test of classical nucleation theory and mean first-passage time formalism on crystallization in the Lennard-Jones liquid. J. Chem. Phys. 131, 104503 (2009).
Davidchack, R. L. Hard spheres revisited: Accurate calculation of the solid–liquid interfacial free energy. J. Chem. Phys. 133, 234701 (2010).
Evans, R. M. L., Poon, W. C. K. & Renth, F. Classification of ordering kinetics in three-phase systems. Phys. Rev. E 64, 031403 (2001).
Polin, M., Grier, D. G. & Han, Y. Colloidal electrostatic interactions near a conducting surface. Phys. Rev. E 76, 041406 (2007).
Peng, Y., Wang, Z., Alsayed, A. M., Yodh, A. G. & Han, Y. Melting of colloidal crystal films. Phys. Rev. Lett. 104, 205703 (2010).
Peng, Y., Wang, Z., Alsayed, A. M., Yodh, A. G. & Han, Y. Melting of multilayer colloidal crystals confined between two walls. Phys. Rev. E 83, 011404 (2011).
Jiang, H., Wada, H., Yoshinaga, N. & Sano, M. Manipulation of colloids by a nonequilibrium depletion force in a temperature gradient. Phys. Rev. Lett. 102, 208301 (2009).
Acknowledgements
This work was supported by Chinese grants NSFC11374248 (Y.H.), NSFC11004143, NSFC21174101, NSFC91027040 and NBRPC.2012CB821500 (Z.Z.), and by US grants NSF DMR12-05463, NSF-MRSEC DMR11-20901 and NASA NNX08AO0G (A.G.Y.).
Author information
Authors and Affiliations
Contributions
Y.P. and Y.H. conceived and designed the research plan. Y.P. carried out the experiment and data analysis with help from Z.W. F.W., Y.P. and Y.H. carried out the theoretical modelling. A.M.A. and Z.Z. synthesized the particles. Y.H., Y.P. and A.G.Y. wrote the paper. Y.H. and A.G.Y. supervised and supported the work. All authors discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3743 kb)
Supplementary Movie 1
Supplementary Movie 1 (MPG 18006 kb)
Supplementary Movie 2
Supplementary Movie 2 (MPG 35160 kb)
Supplementary Movie 3
Supplementary Movie 3 (MPG 13448 kb)
Supplementary Movie 4
Supplementary Movie 4 (MPG 31354 kb)
Supplementary Movie 5
Supplementary Movie 5 (MPG 17250 kb)
Supplementary Movie 6
Supplementary Movie 6 (MPG 38042 kb)
Rights and permissions
About this article
Cite this article
Peng, Y., Wang, F., Wang, Z. et al. Two-step nucleation mechanism in solid–solid phase transitions. Nature Mater 14, 101–108 (2015). https://doi.org/10.1038/nmat4083
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4083
This article is cited by
-
Amyloid formation as a protein phase transition
Nature Reviews Physics (2023)
-
In situ observation of coalescence of nuclei in colloidal crystal-crystal transitions
Nature Communications (2023)
-
Two-step crystallisation in a 2D active magnetic granular system confined by a parabolic potential
Scientific Reports (2023)
-
Lithium crystallization at solid interfaces
Nature Communications (2023)
-
Polymorphic crystalline wetting layers on crystal surfaces
Nature Physics (2023)