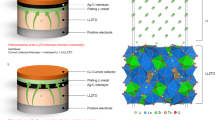
Abstract
Rechargeable lithium, sodium and aluminium metal-based batteries are among the most versatile platforms for high-energy, cost-effective electrochemical energy storage. Non-uniform metal deposition and dendrite formation on the negative electrode during repeated cycles of charge and discharge are major hurdles to commercialization of energy-storage devices based on each of these chemistries. A long-held view is that unstable electrodeposition is a consequence of inherent characteristics of these metals and their inability to form uniform electrodeposits on surfaces with inevitable defects. We report on electrodeposition of lithium in simple liquid electrolytes and in nanoporous solids infused with liquid electrolytes. We find that simple liquid electrolytes reinforced with halogenated salt blends exhibit stable long-term cycling at room temperature, often with no signs of deposition instabilities over hundreds of cycles of charge and discharge and thousands of operating hours. We rationalize these observations with the help of surface energy data for the electrolyte/lithium interface and impedance analysis of the interface during different stages of cell operation. Our findings provide support for an important recent theoretical prediction that the surface mobility of lithium is significantly enhanced in the presence of lithium halide salts. Our results also show that a high electrolyte modulus is unnecessary for stable electrodeposition of lithium.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Armand, M. & Tarascon, J-M. Building better batteries. Nature 451, 652–657 (2008).
Yang, P. & Tarascon, J-M. Towards systems materials engineering. Nature Mater. 11, 560–563 (2012).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4417 (2004).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009).
Morcrette, M. et al. A reversible copper extrusion-insertion electrode for rechargeable Li batteries. Nature Mater. 2, 755–761 (2003).
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J-M. & Schalkwijk, W. V. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Whittingham, M. S. Materials challenges facing electrical energy storage. Mater. Res. Bull. 33, 411–419 (2008).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J-M. Li–O2 and Li–S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Jayaprakash, N., Shen, J., Moganty, S. S., Corona, A. & Archer, L. A. Porous hollow carbon@sulfur composites for high-power lithium–sulfur batteries. Angew. Chem. Int. Ed. 50, 5904–5908 (2011).
Tarascon, J-M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nature Mater. 9, 504–510 (2010).
Lu, Y., Korf, K., Kambe, Y., Tu, Z. & Archer, L. A. Ionic-liquid–nanoparticle hybrid electrolytes: Applications in lithium metal batteries. Angew. Chem. Int. Ed. 53, 488–492 (2014).
Lu, Y., Das, S. K., Moganty, S. S. & Archer, L. A. Ionic liquid–nanoparticle hybrid electrolytes and their application in secondary lithium-metal batteries. Adv. Mater. 24, 4430–4435 (2012).
Tu, Z., Kambe, Y., Lu, Y. & Archer, L. A. Nanoporous polymer-ceramic composite electrolytes for lithium metal batteries. Adv. Energy Mater. 4, 1300654 (2014).
Schaefer, J. L., Yanga, D. A. & Archer, L. A. High lithium transference number electrolytes via creation of 3-dimensional, charged, nanoporous networks from dense functionalized nanoparticle composites. Chem. Mater. 25, 834–839 (2013).
Moganty, S. S. et al. Ionic liquid-tethered nanoparticle suspensions: A novel class of ionogels. Chem. Mater. 24, 1386–1392 (2012).
Moganty, S. S., Jayaprakash, N., Nugent, J. L., Shen, J. & Archer, L. A. Ionic-liquid-tethered nanoparticles: Hybrid electrolytes. Angew. Chem. Int. Ed. 49, 9158–9161 (2010).
Stone, G. M. et al. Resolution of the modulus versus adhesion dilemma in solid polymer electrolytes for rechargeable lithium metal batteries. J. Electrochem. Soc. 159, A222–A227 (2012).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396–A404 (2005).
Nugent, J. L., Moganty, S. S. & Archer, L. A. Nanoscale organic hybrid electrolytes. Adv. Mater. 22, 3677–3680 (2010).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Chazalviel, J-N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 42, 7355–7367 (1990).
Rosso, M., Gobron, T., Brissot, C., Chazalviel, J-N. & Lascaud, S. Onset of dendritic growth in lithium/polymer cells. J. Power Sources 97–98, 804–806 (2001).
Brissot, C., Rosso, M., Chazalviel, J-N. & Lascaud, S. Dendritic growth mechanisms in lithium/polymer cells. J. Power Sources 81–82, 925–929 (1999).
De Jonghe, L. C., Feldman, L. & Millett, P. Some geometrical aspects of breakdown of sodium beta alumina. Mater. Res. Bull. 14, 589–595 (1979).
Ansell, R. The chemical and electrochemical stability of beta-alumina. J. Mater. Sci. 21, 365–379 (1986).
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Ling, C., Banerjee, D. & Matsui, M. Study of the electrochemical deposition of Mg in the atomic level: Why it prefers the non-dendritic morphology. Electrochim. Acta 76, 270–274 (2012).
Gunceler, D., Letchworth-Weaver, K., Sundararaman, R., Schwarz, K. A. & Arias, T. A. The importance of nonlinear fluid response in joint density-functional theory studies of battery systems. Model. Simul. Mater. Sci. Eng. 21, 074005 (2013).
Gunceler, D., Schwarz, K. A., Sundararaman, R., Letchworth-Weaver, K. & Arias, T. A. 16th International Workshop on Computation Physics and Materials Science: Total Energy and Force Methods (International Centre for Theoretical Physics, 2012).
Khurana, R., Schaefer, J. L., Archer, L. A. & Coates, G. W. Suppression of lithium dendrite growth using cross-linked polyethylene/polyethylene oxide electrolytes: A new approach for practical lithium-metal polymer batteries. J. Am. Chem. Soc. 136, 7395–7402 (2014).
Jones, J., Anouti, M., Caillon-Caravanier, M., Willmann, P. & Lemordant, D. Lithium fluoride dissolution equilibria in cyclic alkylcarbonates and water. J. Mol. Liq. 153, 146–152 (2010).
Tikekar, M. D., Archer, L. A. & Koch, D. L. Stability analysis of electrodeposition across a structured electrolyte with immobilized anions. J. Electrochem. Soc. 161, A847–A855 (2014).
Ensling, D., Stjerndahl, M., Nyt’en, A., Gustafsson, T. & Thomas, J. O. A comparative XPS surface study of Li2FeSiO4/C cycled with LiTFSI- and LiPF6-based electrolytes. J. Mater. Chem. 19, 82–88 (2009).
Wagner, C., Riggs, W., Davis, L., Moulder, J. & Muilenberg, G. Handbook of X-ray Photoelectron Spectroscopy (Perkin-Elmer, 1979).
Sotomura, T., Adachi, K., Taguchi, M., Tatsuma, T. & Oyama, N. Developing stable, low impedance interface between metallic lithium anode and polyacrylonitrile-based polymer gel electrolyte by preliminary voltage cycling. J. Power Sources 81, 192–199 (1999).
Brousse, T. et al. All oxide solid-state lithium-ion cells. J. Power Sources 68, 412–415 (1997).
Nakahara, K., Nakajima, R., Matsushima, T. & Majima, H. Preparation of particulate Li4Ti5O12 having excellent characteristics as an electrode active material for power storage cells. J. Power Sources 117, 131–136 (2003).
Lu, Y., Moganty, S. S., Schaefer, J. L. & Archer, L. A. Ionic liquid–nanoparticle hybrid electrolytes. J. Mater. Chem. 22, 4066–4072 (2012).
Acknowledgements
This material is based on work supported as part of the Energy Materials Center at Cornell, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DESC0001086. This work made use of the electrochemical characterization facilities of the KAUST-CU Center for Energy and Sustainability, which is supported by the King Abdullah University of Science and Technology (KAUST) through Award number KUS-C1-018-02. Y.L. thanks J. Jiang and C. Ober in the department of Material Science & Engineering at Cornell University for help with contact angle measurements. The thick LTO electrodes were produced at the US Department of Energy’s (DOE) Cell Fabrication Facility, Argonne National Laboratory. The Cell Fabrication Facility is fully supported by the DOE Vehicle Technologies Program (VTP) within the core funding of the Applied Battery Research (ABR) for Transportation Program.
Author information
Authors and Affiliations
Contributions
Y.L. and L.A.A. conceived the experiments reported in the manuscript. Y.L. performed all studies of the liquid electrolytes. These results are presented in Figs 1a,2a–c,3,4 and 6. Z.T. performed experiments in which liquid electrolytes are infused in nanoporous alumina. These results are reported in Figs 1b and 2d. Y.L. and Z.T. performed the SEM and EDX analyses (Figs 2e–g and 5). Z.T. carried out the XPS analysis in the Supplementary Information. Y.L. and L.A.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5734 kb)
Rights and permissions
About this article
Cite this article
Lu, Y., Tu, Z. & Archer, L. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nature Mater 13, 961–969 (2014). https://doi.org/10.1038/nmat4041
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4041
This article is cited by
-
Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries
Nature Energy (2024)
-
Lignin-reinforced PVDF electrolyte for dendrite-free quasi-solid-state Li metal battery
Rare Metals (2024)
-
Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode
Rare Metals (2024)
-
Enabling an Inorganic-Rich Interface via Cationic Surfactant for High-Performance Lithium Metal Batteries
Nano-Micro Letters (2024)
-
From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
Nano-Micro Letters (2024)