Abstract
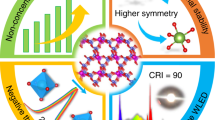
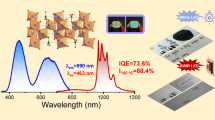
To facilitate the next generation of high-power white-light-emitting diodes (white LEDs), the discovery of more efficient red-emitting phosphor materials is essential. In this regard, the hardly explored compound class of nitridoaluminates affords a new material with superior luminescence properties. Doped with Eu2+, Sr[LiAl3N4] emerged as a new high-performance narrow-band red-emitting phosphor material, which can efficiently be excited by GaN-based blue LEDs. Owing to the highly efficient red emission at λmax ~ 650 nm with a full-width at half-maximum of ~1,180 cm−1 (~50 nm) that shows only very low thermal quenching (>95% relative to the quantum efficiency at 200 °C), a prototype phosphor-converted LED (pc-LED), employing Sr[LiAl3N4]:Eu2+ as the red-emitting component, already shows an increase of 14% in luminous efficacy compared with a commercially available high colour rendering index (CRI) LED, together with an excellent colour rendition (Ra8 = 91, R9 = 57). Therefore, we predict great potential for industrial applications in high-power white pc-LEDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Che, C. & Liu, R-S. Advances in phosphors for light-emitting diodes. J. Phys. Chem. Lett. 2, 1268–1277 (2011).
He, X-H. et al. Dependence of luminescence properties on composition of rare-earth activated (oxy)nitrides phosphors for white-LEDs applications. J. Mater. Sci. 44, 4763–4775 (2009).
Höppe, H. A. Recent developments in the field of inorganic phosphors. Angew. Chem. Int. Ed. 48, 3572–3582 (2009).
Mikami, M. et al. New phosphors for white LEDs: Material design concepts. IOP Conf. Ser.: Mater. Sci. Eng. 1, 012002 (2009).
Xie, R-J. & Hirosaki, N. Silicon-based oxynitride phosphors for white LEDs—A review. Sci. Technol. Adv. Mater. 8, 588–600 (2007).
Xie, R-J. et al. Rare-earth activated nitride phosphors: synthesis, luminescence and applications. Materials 3, 3777–3793 (2010).
Ye, S. et al. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R 71, 1–34 (2010).
Krames, M. et al. Wavelength conversion for producing white light from a high power blue light emitting diode (LED). PCT Int. Appl., WO 2010131133A1 (2010)
Mueller-Mach, R. et al. High-power phosphor-converted light-emitting diodes based on III-nitrides. IEEE J. Sel. Top. Quantum Electron. 8, 339–345 (2002).
Setlur, A. A. Phosphors for LED-based solid-state lighting. Electrochem. Soc. Interf. 18, 32–36 (2009).
Robbins, D. J. The effects of crystal field and temperature on the photoluminescence excitation efficiency of Ce3+ in YAG. J. Electrochem. Soc. 126, 1150–1555 (1979).
Nakamura, S. & Fasol, G. The Blue Laser Diode (Springer, 1997).
Nakamura, S. Present performance of InGaN-based blue/green/yellow LEDs. Proc. SPIE 3002, 26–35 (1997).
Schlotter, P., Schmidt, R. & Schneider, J. Luminescence conversion of blue light emitting diodes. Appl. Phys. A 64, 417–418 (1997).
Krames, M. R. et al. Status and future of high-power light-emitting diodes for solid-state lighting. J. Disp. Tech. 3, 160–175 (2007).
Höppe, H. A. et al. Luminescence in Eu2+-doped Ba2Si5N8: Fluorescence, thermoluminescence and upconversion. J. Phys. Chem. Solids 61, 2001–2006 (2000).
Mueller-Mach, R. et al. Highly efficient all-nitride phosphor-converted white light emitting diode. Phys. Status Solidi 202, 1727–1732 (2005).
Uheda, K., Hirosaki, N. & Yamamoto, H. Host lattice materials in the system Ca3N2–AlN–Si3N4 for white light emitting diode. Phys. Status Solidi 11, 2712–2717 (2006).
Uheda, K. et al. Luminescence properties of a red phosphor, CaAlSiN3:Eu2+, for white light-emitting diodes. Electrochem. Solid State Lett. 9, H22–H25 (2006).
Mueller-Mach, R. & Mueller, G. White light emitting diodes for illumination. Proc. SPIE 3938, 30–41 (2000).
Zeuner, M., Pagano, S. & Schnick, W. Nitridosilicates and oxonitridosilicates: From ceramic materials to structural and functional diversity. Angew. Chem. Int. Ed. 50, 7754–7775 (2011).
Blasse, G. & Bril, A. Characteristic luminescence II. Efficiency of phosphors excited in the activator. Philips Tech. Rev. 31, 314–323 (1970).
Vos, J. J. Colorimetric and photometric properties of a 2-deg fundamental observer. Color Res. Appl. 3, 125–128 (1978).
Yamashita, N., Harada, O. & Nakamura, K. Photoluminescence spectra of Eu2+ centers in Ca(S, Se):Eu and Sr(S, Se):Eu. Jpn. J. Appl. Phys. 34, 5539–5545 (1995).
MacAdam, D. L. Visual sensitivities to color differences in daylight. J. Opt. Soc. Am. 32, 247–273 (1942).
Stoll, H. & Hoppe, R. Ein neues Oxoplumbat (IV): CsNa3[PbO4]. Rev. Chim. Min. 24, 96–115 (1987).
Liebau, F. Structural Chemistry of Silicates (Springer, 1985).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Scheu, C. et al. Electron energy-loss near-edge structure of metal-alumina interfaces. Microsc. Microanal. Microstruct. 6, 19–31 (1995).
Mauchamp, V. et al. Determination of lithium insertion sites in LixTiP4 (x = 2–11) by electron energy-loss spectroscopy. J. Phys. Chem. C 111, 3996–4002 (2007).
Zeuner, M. et al. Li2CaSi2N4 and Li2SrSi2N4—A synthetic approach to three-dimensional lithium nitridosilicates. Eur. J. Inorg. Chem. 4945–4591 (2010).
Lupart, S. & Schnick, W. LiCa3Si2N5—A lithium nitridosilicate with a [Si2N5]7− double-chain. Z. Anorg. Allg. Chem. 638, 2015–2019 (2012).
Pagano, S. et al. Single-crystal structure determination and solid-state NMR investigations of lithium nitridosilicate Li2SiN2 synthesized by a precursor approach employing amorphous “Si(CN2)2”. Eur. J. Inorg. Chem. 1579–1584 (2009).
Henderson, B. & Imbusch, G. F. Optical Spectroscopy of Inorganic Solids (Oxford Univ. Press, 2006).
Schlieper, T., Milius, W. & Schnick, W. Nitrido-silicate. II. Hochtemperatur-Synthesen und Kristallstrukturen von Sr2Si5N8 und Ba2Si5N8 . Z. Anorg. Allg. Chem. 621, 1380–1384 (1995).
Botterman, J. et al. Mechanoluminescence in BaSi2O2N2:Eu. Acta Mater. 60, 5494–5500 (2012).
Kechele, J. A. et al. Structure elucidation of BaSi2O2N2—A host lattice for rare-earth doped luminescent materials in phosphor-converted (pc)-LEDs. Solid State Sci. 11, 537–543 (2009).
Li, Y. Q. et al. Luminescence properties of Eu2+-activated alkaline-earth silicon-oxynitride MSi2O2−δN2+2/3δ . Chem. Mater. 17, 3242–3248 (2005).
Seibald, M. et al. Highly efficient pc-LED phosphors Sr1−xBaxSi2O2N2:Eu2+ (0 ≤ x ≤ 1)—crystal structures and luminescence properties revisited. Crit. Rev. Solid State Mater. Sci. 39, 215–229 (2014).
Wang, L. et al. Electronic structure and linear optical property of BaSi2N2O2 crystal. J. Alloys Compd 509, 10203–10206 (2011).
Yun, B-G. et al. Preparation and luminescence properties of single-phase BaSi2O2N2:Eu2+, a bluish-green phosphor for white light-emitting diodes. J. Electrochem. Soc. 157, J364–J370 (2010).
Meijerink, A. & Blasse, G. Luminescence properties of Eu2+-activated alkaline-earth haloborates. J. Lumin. 43, 283–289 (1989).
Dirksen, G. J. & Blasse, G. Luminescence in the pentaborate LiBa2B5O10 . J. Solid State Chem. 92, 591–593 (1991).
Dorenbos, P. A review on how lanthanide impurity levels change with chemistry and structure of inorganic compounds. J. Solid State Sci. Technol. 2, R3001–R3011 (2013).
Dorenbos, P. 5d-level energies of Ce3+ and the crystalline environment. I. Fluoride compounds. Phys. Rev. B 62, 15640–15649 (2000).
Dorenbos, P. 5d-level energies of Ce3+ and the crystalline environment. III. Oxides containing ionic complexes. Phys. Rev. B 64, 125117 (2001).
Dorenbos, P. Relating the energy of the [Xe]5d1 configuration of Ce3+ in inorganic compounds with anion polarizability and cation electronegativity. Phys. Rev. B 65, 235110 (2002).
Li, Y. Q. et al. Luminescence properties of red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) LED conversion phosphors. J. Alloys Compd 417, 273–279 (2006).
Spek, A. L. PLATON—A Multipurpose Crystallographic Tool, v1.07 (Utrecht Univ., 2003).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Stoe & Cie GmbH, WINXPOW—Program for Powder Data Handling, v2.21 (Darmstadt, 2007).
Coelho, A. TOPAS—Academic 2007 (Coelho Software)
Egerton, R. F. Electron Energy-Loss Spectroscopy in the Electron Microscope 2nd edn (Plenum Press, 1996).
Harris, R. K. et al. NMR nomenclature: Nuclear spin properties and conventions for chemical shifts: IUPAC recommendations 2001. Solid State Nucl. Magn. Reson. 22, 458–483 (2002).
Acknowledgements
The authors thank T. Miller and C. Hoch for recording the single-crystal X-ray data and C. Minke for performing the EDX and solid-state NMR measurements (all at LMU Munich). We would like to thank P. Huppertz and H. Ohland, Philips Technologie GmbH Lumileds Development Center Aachen for luminescence measurements. Furthermore the authors gratefully acknowledge financial support from the Fonds der Chemischen Industrie (FCI).
Author information
Authors and Affiliations
Contributions
P.P., P.J.S. and W.S. conceived and designed the project. P.P. performed the bulk of the experimental work with significant help from V.W., C.H., A.T. and A-K.H. All authors participated in measurements and analysis of the data, discussed the results and took part in producing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1328 kb)
Rights and permissions
About this article
Cite this article
Pust, P., Weiler, V., Hecht, C. et al. Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nature Mater 13, 891–896 (2014). https://doi.org/10.1038/nmat4012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4012
This article is cited by
-
Red/deep-red emitting phosphors of Eu3+/Dy3+ co-doped NaSrGd(WO4)3 for LED and optical anti-counterfeiting
Applied Physics A (2024)
-
Exploring the crystal structure and luminescence properties of green-emitting Tb3+ activated vanadate nanocrystals for potential indoor lighting applications
Optical and Quantum Electronics (2024)
-
A study of virescent emission from Er3+-activated ternary gadolinium-based nanophosphor system applicable for current pc-WLEDs and solid-state lightings
Indian Journal of Physics (2024)
-
A guide to comprehensive phosphor discovery for solid-state lighting
Nature Reviews Materials (2023)
-
Modification of the green CSS:Ce3+ phosphor by ion substitution for obtaining an orange phosphor for white LEDs
Journal of Materials Science: Materials in Electronics (2023)