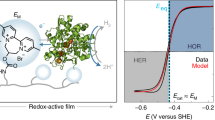
Abstract
Molecular switches gate many fundamental processes in natural and artificial systems. Here, we report the development of an electrochemical platform in which a proton carrier switches the activity of a catalyst. By incorporating an alkyl phosphate in the lipid layer of a hybrid bilayer membrane, we regulate proton transport to a Cu-based molecular oxygen reduction reaction catalyst. To construct this hybrid bilayer membrane system, we prepare an example of a synthetic Cu oxygen reduction reaction catalyst that forms a self-assembled monolayer on Au surfaces. We then embed this Cu catalyst inside a hybrid bilayer membrane by depositing a monolayer of lipid on the self-assembled monolayer. We envisage that this electrochemical system can give a unique mechanistic insight not only into the oxygen reduction reaction, but into proton-coupled electron transfer in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Browne, W. R. & Feringa, B. L. Making molecular machines work. Nature Nanotech. 1, 25–35 (2006).
Balzani, V., Credi, A., Raymo, F. M. & Stoddart, J. F. Artificial molecular machines. Angew. Chem. Int. Ed. 39, 3348–3391 (2000).
Hirjibehedin, C. F. et al. Large magnetic anisotropy of a single atomic spin embedded in a surface molecular network. Science 317, 1199–1203 (2007).
Fuentes, N. et al. Organic-based molecular switches for molecular electronics. Nanoscale 3, 4003–4014 (2011).
Alberts, B. et al. Molecular Biology of the Cell 4th edn (Garland Science, 2002).
Von Lintig, J., Kiser, P. D., Golczak, M. & Palczewski, K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 (2010).
Donaldson, J. & Segev, N. Trafficking Inside Cells: Pathways, Mechanisms and Regulation (Landes Bioscience and Springer Science, 2009).
Lee, H. J., Gennis, R. B. & Ädelroth, P. Entrance of the proton pathway in cbb3-type heme-copper oxidases. Proc. Natl Acad. Sci. USA 108, 17661–17666 (2011).
Mayer, J. M. Proton-coupled electron transfer: A reaction chemist’s view. Annu. Rev. Phys. Chem. 55, 363–390 (2004).
Chen, Z., Vannucci, A. K., Concepcion, J. J., Jurss, J. W. & Meyer, T. J. Proton-coupled electron transfer at modified electrodes by multiple pathways. Proc. Natl Acad. Sci. USA 108, E1461–E1469 (2011).
Huynh, M. H. V. & Meyer, T. J. Proton-coupled electron transfer. Chem. Rev. 107, 5004–5064 (2007).
Wenger, O. S. Proton-coupled electron transfer with photoexcited metal complexes. Acc. Chem. Res. 46, 1517–1526 (2013).
Thorseth, M. A., Tornow, C. E., Tse, E. C. M. & Gewirth, A. A. Cu complexes that catalyze the oxygen reduction reaction. Coord. Chem. Rev. 257, 130–139 (2013).
Boulatov, R., Collman, J. P., Shiryaeva, I. M. & Sunderland, C. J. Functional analogues of the dioxygen reduction site in cytochrome oxidase: mechanistic aspects and possible effects of CuB . J. Am. Chem. Soc. 124, 11923–11935 (2002).
Thorseth, M. A., Letko, C. S., Rauchfuss, T. B. & Gewirth, A. A. Dioxygen and hydrogen peroxide reduction with hemocyanin model complexes. Inorg. Chem. 50, 6158–6162 (2011).
Oberst, J. L., Thorum, M. S. & Gewirth, A. A. Effect of pH and azide on the oxygen reduction reaction with a pyrolyzed Fe phthalocyanine catalyst. J. Phys. Chem. C 116, 25257–25261 (2012).
Rosenthal, J. & Nocera, D. G. Role of proton-coupled electron transfer in O–O bond activation. Acc. Chem. Res. 40, 543–553 (2007).
Chng, L. L., Chang, C. J. & Nocera, D. G. Catalytic O–O activation chemistry mediated by iron hangman porphyrins with a wide range of proton-donating abilities. Org. Lett. 5, 2421–2424 (2003).
Thorseth, M. A., Letko, C. S., Tse, E. C. M., Rauchfuss, T. B. & Gewirth, A. A. Ligand effects on the overpotential for dioxygen reduction by tris(2-pyridylmethyl)amine derivatives. Inorg. Chem. 52, 628–634 (2013).
Hosseini, A. et al. Hybrid bilayer membrane: A platform to study the role of proton flux on the efficiency of oxygen reduction by a molecular electrocatalyst. J. Am. Chem. Soc. 133, 11100–11102 (2001).
Plant, A. L. Self-assembled phospholipid/alkanethiol biomimetic bilayers on gold. Langmuir 9, 2764–2767 (1993).
Plant, A. L. Supported hybrid bilayer membranes as rugged cell membrane mimics. Langmuir 15, 5128–5135 (1999).
Twardowski, M. & Nuzzo, R. G. Molecular recognition at model organic interfaces: Electrochemical discrimination using self-assembled monolayers (SAMs) modified via the fusion of phospholipid vesicles. Langmuir 19, 9781–9791 (2003).
Twardowski, M. & Nuzzo, R. G. Phase dependent electrochemical properties of polar self-assembled monolayers (SAMs) modified via the fusion of mixed phospholipid vesicles. Langmuir 20, 175–180 (2004).
Thorum, M. S., Yadav, J. & Gewirth, A. A. Oxygen reduction activity of a copper complex of 3,5-diamino-1,2,4-triazole supported on carbon black. Angew. Chem. Int. Ed. 48, 165–167 (2009).
Devaraj, N. K., Decreau, R. A., Ebina, W., Collman, J. P. & Chidsey, C. E. D. Rate of interfacial electron transfer through the 1,2,3-triazole linkage. J. Phys. Chem. B 110, 15955–15962 (2006).
Inman, C. E., Reed, S. M. & Hutchison, J. E. In situ deprotection and assembly of s-tritylalkanethiols on gold yields monolayers comparable to those prepared directly from alkanethiols. Langmuir 20, 9144–9150 (2004).
Ermakova, T. G. et al. Polarographic reduction of 1-substituted 1,2,4-triazoles. Chem. Heterocyc. Compd. 16, 313–315 (1980).
Hosseini, A. et al. Ferrocene embedded in an electrode-supported hybrid lipid bilayer membrane: A model system for electrocatalysis in a biomimetic environment. Langmuir 26, 17674–17678 (2010).
Rowe, G. K. & Creager, S. E. Interfacial solvation and double-layer effects on redox reactions in organized assemblies. J. Phys. Chem. 98, 5500–5507 (1994).
Subczynski, W. K. & Hyde, J. S. Concentration of oxygen in lipid bilayers using a spin-label method. Biophys. J. 41, 283–286 (1983).
Windrem, D. A. & Plachy, W. Z. The diffusion-solubility of oxygen in lipid bilayers. Biochim. Biophys. Acta 600, 655–665 (1980).
Chang, P. & Wilke, C. R. Some measurements of diffusion in liquids. J. Phys. Chem. 59, 592–596 (1955).
Jain, M. K. Introduction to Biological Membranes 2nd edn (Wiley, 1988).
Collman, J. P. et al. A cytochrome c oxidase model catalyzes oxygen to water reduction under rate-limiting electron flux. Science 315, 1565–1568 (2007).
Collman, J. P. et al. Role of a distal pocket in the catalytic O2 reduction by cytochrome c oxidase models immobilized on interdigitated array electrodes. Proc. Natl Acad. Sci. USA 106, 7320–7323 (2009).
Srivastava, A., Singh, S. & Krishnamoorthy, G. Rapid transport of protons across membranes by aliphatic amines and acids. J. Phys. Chem. 99, 11302–11305 (1995).
Schönfeld, P., Schild, L. & Kunz, W. Long-chain fatty acids act as protonophoric uncouplers of oxidative phosphorylation in rat liver mitochondria. Biochim. Biophys. Acta 977, 266–272 (1989).
McConnell, H. M. & Kornberg, R. D. Inside–outside transitions of phospholipids in vesicle membranes. Biochemistry 10, 1111–1120 (1971).
Palermo, E. F., Lee, D-K., Ramamoorthy, A. & Kuroda, K. Role of cationic group structure in membrane binding and disruption by amphiphilic copolymers. J. Phys. Chem. B 115, 366–375 (2010).
Albrecht, O., Gruler, H. & Sackmann, E. Polymorphism of phospholipid monolayers. J. Phys. France 39, 301–313 (1978).
John, K., Schreiber, S., Kubelt, J., Herrmann, A. & Müller, P. Transbilayer movement of phospholipids at the main phase transition of lipid membranes: Implications for rapid flip–flop in biological membranes. Biophys. J. 83, 3315–3323 (2002).
Han, X., Wang, L., Qi, B., Yang, X. & Wang, E. A strategy for constructing a hybrid bilayer membrane based on a carbon substrate. Anal. Chem. 75, 6566–6570 (2003).
Mercado, F. V., Maggio, R. & Wilke, N. Phase diagram of mixed monolayers of stearic acid and dimyristoylphosphatidylcholine. Effect of the acid ionization. Chem. Phys. Lipids 164, 386–392 (2011).
Mercado, F. V., Maggio, R. & Wilke, N. Modulation of the domain topography of biphasic monolayers of stearic acid and dimyristoyl phosphatidylcholine. Chem. Phys. Lipids 165, 232–237 (2012).
Gong, K., Du, F., Xia, Z., Durstock, M. & Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323, 760–764 (2009).
Acknowledgements
C.J.B. acknowledges a National Science Foundation Graduate Research Fellowship (NSF DGE-1144245) and a Springborn Fellowship. E.C.M.T. acknowledges a Croucher Foundation Scholarship. We thank Michael Cason for his assistance in preparing Au on glass substrates. We thank the US Department of Energy (DE-FG02-95ER46260) for support of this research. This work was carried out in part in the Frederick Seitz Materials Research Laboratory Central Facilities, which are partially supported by the US Department of Energy (DE-FG02-07ER46453 and DE-FG02-07ER46471).
Author information
Authors and Affiliations
Contributions
C.J.B., E.C.M.T., S.C.Z., A.H. and A.A.G. designed the experiments. C.J.B., E.C.M.T. and T.B.S. performed the experiments. Y.L. synthesized BTT. C.J.B., E.C.M.T., Y.L., S.C.Z., A.H. and A.A.G. wrote the paper. C.J.B., E.C.M.T., A.H. and A.A.G. analysed the data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2007 kb)
Rights and permissions
About this article
Cite this article
Barile, C., Tse, E., Li, Y. et al. Proton switch for modulating oxygen reduction by a copper electrocatalyst embedded in a hybrid bilayer membrane. Nature Mater 13, 619–623 (2014). https://doi.org/10.1038/nmat3974
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3974
This article is cited by
-
Hybrid bilayer membranes as platforms for biomimicry and catalysis
Nature Reviews Chemistry (2022)
-
Molecular enhancement of heterogeneous CO2 reduction
Nature Materials (2020)
-
Shape-Controlled Synthesis of Luminescent Hemoglobin Capped Hollow Porous Platinum Nanoclusters and their Application to Catalytic Oxygen Reduction and Cancer Imaging
Scientific Reports (2018)
-
Molecular electrocatalysts for the oxygen reduction reaction
Nature Reviews Chemistry (2017)
-
Bioinspired catalytic materials for energy-relevant conversions
Nature Energy (2017)