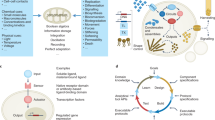
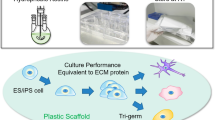
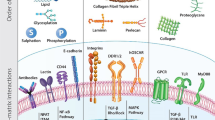
Abstract
Polymeric substrates are being identified that could permit translation of human pluripotent stem cells from laboratory-based research to industrial-scale biomedicine. Well-defined materials are required to allow cell banking and to provide the raw material for reproducible differentiation into lineages for large-scale drug-screening programs and clinical use. Yet more than 1 billion cells for each patient are needed to replace losses during heart attack, multiple sclerosis and diabetes. Producing this number of cells is challenging, and a rethink of the current predominant cell-derived substrates is needed to provide technology that can be scaled to meet the needs of millions of patients a year. In this Review, we consider the role of materials discovery, an emerging area of materials chemistry that is in large part driven by the challenges posed by biologists to materials scientists.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocytes. Science 282, 1145–1147 (1998).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Rajamohan, D., Matsa, E. & Kalra, S. Current status of drug screening and disease modelling in human pluripotent stem cells. BioEssays 35, 281–298 (2012).
Thomas, R. J. et al. Automated, scalable culture of human embryonic stem cells in feeder-free conditions. Biotechnol. Bioeng. 102, 1636–1644 (2009).
Mahlstedt, M. M. et al. Maintenance of pluripotency in human embryonic stem cells cultured on a synthetic substrate in conditioned medium. Biotechnol. Bioeng. 105, 130–140 (2010).
Xu, C. et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 19, 971–974 (2001).
Martin, M. J., Muotri, A., Gage, F. & Varki, A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nature Med. 11, 228–232 (2005).
Kleinman, H. K. et al. Isolation and characterization of type-IV procollagen, laminin, and heparin-sulfate proteoglycans from EHS sarcoma. Biochemistry 21, 6188–6193 (1982).
Jin, S., Yao, H., Weber, J. L., Melkoumian, Z. K. & Ye, K. A synthetic, xeno-free peptide surface for expansion and directed differentiation of human induced pluripotent stem cells. PLoS ONE 7, e50880 (2012).
Nagaoka, M., Si-Tayeb, K., Akaike, T. & Duncan, S. A. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev. Biol. 10, 60 (2010).
Stelzer, T., Marwood, T. & Neeley, C. Innovative animal component-free surface for the cultivation of human embryonic stem cells. BMC Proc. 5 (Suppl. 8), P51 (2011).
Swistowski, A. et al. Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS ONE 4, e6233 (2009).
Ludwig, T. E. et al. Derivation of human embryonic stem cells in defined conditions. Nature Biotechnol. 24, 185–187 (2006).
Wang, L. et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signalling. Blood 110, 4111–4119 (2006).
Bergstrom, R., Strom, S., Holm, F., Feki, A. & Hovatta, O. Xeno-free culture of human pluripotent stem cells. Methods Mol. Biol. 767, 125–136 (2011).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nature Methods 8, 424–429 (2011).
Li, Y., Powell, S., Brunette, E., Lebkowski, J. & Mandalam, R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol. Bioeng. 91, 688–698 (2005).
Genbacev, O. et al. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil. Steril. 83, 1517–1529 (2005).
Rajala, K. et al. A defined and xeno-free culture method enabling the establishment of clinical-grade human embryonic, induced pluripotent and adipose stem cells. PLoS ONE 5, e12046 (2010).
Furue, M. K. et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl Acad. Sci. USA 105, 13409–13414 (2008).
Amit, M., Shakiri, C., Margulets, V. & Itskovitz-Eldor, J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 70, 837–845 (2004).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnol. 25, 681–686 (2007).
Xu, Y. et al. Revealing a core signalling regulatory mechanism for pluripotent stem cell survival and self-renewal survival by small molecules. Proc. Natl Acad. Sci. USA 107, 8129–8134 (2010).
Tsutsui, H. et al. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nature Commun. 2, 167 (2011).
Qi, X. et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc. Natl Acad. Sci. USA 101, 6027–6032 (2004).
Damoiseaux, R., Sherman, S. P., Alva, J. A., Peterson, C. & Pyle, A. D. Integrated chemical genomics reveals modifiers of survival in human embryonic stem cells. Stem Cells 27, 533–542 (2009).
Barbaric, I. et al. Novel regulators of stem cell fates identified by a multivariate phenotype screen of small compounds on human embryonic stem cell colonies. Stem Cell Res. 5, 104–119 (2010).
Buehr, M. et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 (2008).
Cai, J. et al. Assessing self-renewal and differentiation in human embryonic stem cell lines. Stem Cells 24, 516–530 (2006).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 10, 55–63 (2004).
Bone, H. K. et al. Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides. Chem. Biol. 16, 15–27 (2009).
Xiong, L. et al. Heat shock protein 90 is involved in regulation of hypoxia-driven proliferation of embryonic neural stem/progenitor cells. Cell Stress Chaperones 14, 183–192 (2009).
Miyabayashi, T., Yamamoto, M., Sato, A., Sakano, S. & Takahashi, Y. Indole derivatives sustain embryonic stem cell self-renewal in long-term culture. Biosci. Biotechnol. Biochem. 72, 1242–1248 (2008).
Chen, L. & Khillan, J. S. Promotion of feeder-independent self-renewal of embryonic stem cells by retinol (vitamin A). Stem Cells 26, 1858–1864 (2008).
Li, M. et al. Neuronal differentiation of C17.2 neural stem cells induced by a natural flavonoid, baicalin. ChemBioChem 12, 449–456 (2011).
Anneren, C., Cowan, C. A. & Melton, D. A. The Src family of tyrosine kinases is important for embryonic stem cell self-renewal. J. Biol. Chem. 279, 31590–31598 (2004).
Miyazaki, T. et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 375, 27–32 (2008).
Miyazaki, T. et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nature Commun. 3, 1236–1245 (2012).
Rodin, S. et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nature Biotechnol. 28, 611–615 (2010).
Derda, R. et al. Defined substrates for human embryonic stem cell growth identified from surface arrays. ACS Chem. Biol. 2, 347–355 (2007).
Melkoumian, Z. et al. Synthetic peptide-acrylate surfaces for the long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nature Biotechnol. 28, 606–610 (2010).
Villa-Diaz, L. G. et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nature Biotechnol. 28, 581–583 (2010).
Irwin, E. E., Gupta, R., Dashti, D. C. & Healy, K. E. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 32, 6912–6919 (2011).
Klim, J. R., Li, L. Y., Wrighton, P. J., Piekarczyk, M. S. & Kiessling L. L. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nature Methods 7, 989–996 (2010).
Anderson, D. G., Putnam, D., Lavik, E. B., Mahmood, T. A. & Langer, R. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials 26, 4892–4897 (2005).
Brocchini, S., James, K., Tangpasuthadol, V. & Kohn, J. A combinatorial approach for polymer design. J. Am. Chem. Soc. 119, 4553–4554 (1997).
Brafman, D. A. et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 31, 9135–9144 (2010).
Anderson, D. G., Levenburg, S. & Langer, R. Nanolitre-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nature Biotechnol. 22, 863–866 (2004).
Mei, Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nature Mater. 9, 768–778 (2010).
Saha, K. et al. Surface-engineered substrates for improved pluripotent stem cell culture under fully defined conditions. Proc. Natl Acad. Sci. USA 108, 18714–18719 (2011).
Zhang, R. et al. A thermoresponsive and chemically defined hydrogel for long-term culture of human embryonic stem cells. Nature Commun. 4, 1335–1345 (2013).
Meng, Y. et al. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J. 24, 1056–1065 (2010).
Harb, N., Archer, T. & Sato, N. The Rho-ROCK-Myosin axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS ONE 3, e3001 (2008).
Rowland, T. J. et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 19, 1231–1240 (2010).
Prowse, A. B. J., Chong, C., Gray, P. P. & Munro, T. P. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 6, 1–12 (2011).
Li, L., Bennett, S. A. L. & Wang, L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adh. Migr. 6, 59–70 (2012).
Koenig, A. L., Gambillara, V. & Grainger, D. W. Correlating fibronectin adsorption with endothelial cell adhesion and signalling on polymer substrates. J. Biomed. Mater. Res. Part A 64, 20–37 (2003).
Weber, N., Bolikal, D., Bourke, S. L. & Kohn, J. Small changes in the polymer structure influence the adsorption behaviour of fibrinogen on polymer surfaces: validation of a new rapid screening technique. J. Biomed. Mater. Res. A 68, 496–503 (2004).
Ingber, D. E. The riddle of morphogenesis: a question of solution chemistry or molecular cell engineering? Cell 75, 1249–1252 (1993).
Wan, L. Q. et al. Geometric control of human stem cell morphology and differentiation. Integr. Biol. 2, 346–353 (2010).
Fu, J. et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature Methods 7, 733–736 (2010).
Trappmann, B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Mater. 27, 642–649 (2012).
Musah, S. et al. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 6, 10168–10177 (2012).
Cranford, S. W., de Boer, J., van Blitterswijk, C. & Buehler, M. J. Materiomics: An -omics approach to biomaterials research. Adv. Mater. 25, 802–824 (2013).
Brocchini, S., James, K., Tangpasuthadol, V. & Kohn, J. Structure–property correlations in a combinatorial library of biodegradable materials. J. Biomed. Mater. Res. 42, 67–75 (1998).
Epa, V. C. et al. Modelling human embryoid body cell adhesion to a combinatorial library of polymer surfaces. J. Mater. Chem. 22, 20902–20906 (2012).
Chilkoti, A., Schmierer, A. E., Pérez-Luna, V. H. & Ratner, B. D. Investigating the relationship between surface chemistry and endothelial cell growth: partial least-squares regression of the static secondary ion mass spectra of oxygen-containing plasma-deposited films. Anal. Chem. 67, 2883–2891 (1995).
Urquhart, A. J. et al. High throughput surface characterisation of a combinatorial material library. Adv. Mater. 19, 2486–2491 (2007).
Urquhart, A. J. et al. TOF-SIMS analysis of a 576 micropatterned copolymer array to reveal surface chemical moieties that control wettability. Anal. Chem. 80, 135–142 (2008).
Martin, S. Lattice enumeration for inverse molecular design using the signature descriptor. J. Chem. Inf. Model. 52, 1787–1797 (2012).
Dixon, J. E. et al. Composite hydrogels that switch human pluripotent stem cells from self-renewal to differentiation. Proc. Natl Acad. Sci. USA 111, 5580–5585 (2014).
Maier, W. F., Stöwe, K. & Sieg, S. Combinatorial and high-throughput materials science. Angew. Chem. Int. Ed. 46, 6016–6067 (2007).
Acknowledgements
C.D. is supported by EPSRC, British Heart Foundation, Heart Research UK and National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). M.R.A. gratefully acknowledges EPSRC (grant number EP/H045384/1) and the Wellcome Trust for funding, and The Royal Society for the provision of his Wolfson Research Merit Award.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Celiz, A., Smith, J., Langer, R. et al. Materials for stem cell factories of the future. Nature Mater 13, 570–579 (2014). https://doi.org/10.1038/nmat3972
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3972
This article is cited by
-
Nanotopography reveals metabolites that maintain the immunomodulatory phenotype of mesenchymal stromal cells
Nature Communications (2023)
-
In vitro, ex vivo, and in vivo models for dental pulp regeneration
Journal of Materials Science: Materials in Medicine (2023)
-
Cell-controlled dynamic surfaces for skeletal stem cell growth and differentiation
Scientific Reports (2022)
-
Evaluation of the relative potential for contact and doffing transmission of SARS-CoV-2 by a range of personal protective equipment materials
Scientific Reports (2022)
-
Validation of a multicellular tumor microenvironment system for modeling patient tumor biology and drug response
Scientific Reports (2021)