Abstract
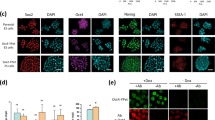
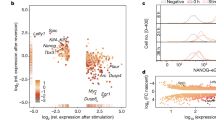
Embryonic stem cells (ESCs) self-renew in a state of naïve pluripotency in which they are competent to generate all somatic cells. It has been hypothesized that, before irreversibly committing, ESCs pass through at least one metastable transition state. This transition would represent a gateway for differentiation and reprogramming of somatic cells. Here, we show that during the transition, the nuclei of ESCs are auxetic: they exhibit a cross-sectional expansion when stretched and a cross-sectional contraction when compressed, and their stiffness increases under compression. We also show that the auxetic phenotype of transition ESC nuclei is driven at least in part by global chromatin decondensation. Through the regulation of molecular turnover in the differentiating nucleus by external forces, auxeticity could be a key element in mechanotransduction. Our findings highlight the importance of nuclear structure in the regulation of differentiation and reprogramming.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Betschinger, J. et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell 153, 335–347 (2013).
Silva, J. & Smith, A. Capturing pluripotency. Cell 132, 532–536 (2008).
Smith, A. Pluripotent stem cells: Private obsession and public expectation. EMBO Mol. Med. 2, 113–116 (2010).
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Young, R. A. Control of the embryonic stem cell state. Cell 144, 940–954 (2011).
Marks, H. et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149, 590–604 (2012).
Loh, K. M. & Lim, B. A precarious balance: Pluripotency factors as lineage specifiers. Cell Stem Cell 8, 363–369 (2011).
Fisher, C. L. & Fisher, A. G. Chromatin states in pluripotent, differentiated, and reprogrammed cells. Curr. Opin. Genet. Dev. 21, 140–146 (2011).
Hanna, J. et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl Acad. Sci. USA 107, 9222–9227 (2010).
Wray, J. et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nature Cell Biol. 13, 838–845 (2011).
Toyooka, Y., Shimosato, D., Murakami, K., Takahashi, K. & Niwa, H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135, 909–918 (2008).
Ying, Q. L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Franze, K. Atomic force microscopy and its contribution to understanding the development of the nervous system. Curr. Opin. Genet. Dev. 21, 530–537 (2011).
Lu, Y. B. et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc. Natl Acad. Sci. USA 103, 17759–17764 (2006).
Mahaffy, R. E., Park, S., Gerde, E., Kas, J. & Shih, C. K. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys J 86, 1777–1793 (2004).
Evans, K. E. & Alderson, A. Auxetic materials: Functional materials and structures from lateral thinking!. Adv Mater 12, 617 (2000).
Trickey, W. R., Baaijens, F. P., Laursen, T. A., Alexopoulos, L. G. & Guilak, F. Determination of the Poisson’s ratio of the cell: Recovery properties of chondrocytes after release from complete micropipette aspiration. J. Biomech. 39, 78–87 (2006).
Moeendarbary, E. et al. The cytoplasm of living cells behaves as a poroelastic material. Nature Mater. 12, 253–261 (2013).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Rowat, A. C. et al. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J. Biol. Chem. 288, 8610–8618 (2013).
Booth-Gauthier, E. A., Alcoser, T. A., Yang, G. & Dahl, K. N. Force-induced changes in subnuclear movement and rheology. Biophys. J. 103, 2423–2431 (2012).
Chowdhury, F. et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature Mater. 9, 82–88 (2010).
Dahl, K. N., Ribeiro, A. J. S. & Lammerding, J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 102, 1307–1318 (2008).
Mazumder, A., Roopa, T., Basu, A., Mahadevan, L. & Shivashankar, G. V. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys. J. 95, 3028–3035 (2008).
Pajerowski, J. D., Dahl, K. N., Zhong, F. L., Sammak, P. J. & Discher, D. E. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl Acad. Sci. USA 104, 15619–15624 (2007).
Meshorer, E. et al. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 10, 105–116 (2006).
Chalut, K. J. et al. Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells. Biophys. J. 103, 2060–2070 (2012).
Masui, O. et al. Live-Cell Chromosome Dynamics and Outcome of X Chromosome Pairing Events during ES Cell Differentiation. Cell. 145, 447–458 (2011).
Blumenfeld, R. & Edwards, S. F. Theory of strains in auxetic materials. J. Supercond. Nov. Magn. 25, 565–571 (2012).
Nichols, J., Silva, J., Roode, M. & Smith, A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 (2009).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Plusa, B., Piliszek, A., Frankenberg, S., Artus, J. & Hadjantonakis, A. K. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development 135, 3081–3091 (2008).
Acknowledgements
This work was supported by the Royal Society, UK Medical Research Council and Wellcome Trust (G.W.W., C.L.F. and K.J.C.), a European Research Council starting grant (S.P. and U.F.K.), a Human Frontier in Science Program grant (R.J.M.F and C.R.M.), a Leverhulme and Newton Trust Early Career Fellowship (S.P.) and the UK Medical Research Council (Career Development Award to K.F.). We also thank T. Kalkan for providing cells and guidance, A. Ekpenyong for experimental support, D. Morrison for assistance with electron microscopy, E. Paluch for critical reading of the manuscript, and A. Brown, A. Smith and J. Nichols for helpful discussions.
Author information
Authors and Affiliations
Contributions
K.J.C. developed the project; K.F., R.J.M.F., U.F.K. and K.J.C. designed the research; S.P., K.F., G.W.W., C.R.M., C.L.F. and K.J.C performed the experiments; S.P., K.F., C.R.M., A.J.K., U.F.K. and K.J.C. analysed and discussed the data; S.P., K.F. and K.J.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2014 kb)
Supplementary Information
Supplementary Movie 1 (WMV 1453 kb)
Supplementary Information
Supplementary Movie 2 (WMV 729 kb)
Rights and permissions
About this article
Cite this article
Pagliara, S., Franze, K., McClain, C. et al. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nature Mater 13, 638–644 (2014). https://doi.org/10.1038/nmat3943
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3943
This article is cited by
-
Non-affinity: The emergence of networks from amorphous planar graphs
Science China Physics, Mechanics & Astronomy (2023)
-
A three step recipe for designing auxetic materials on demand
Communications Physics (2022)
-
LIM Tracker: a software package for cell tracking and analysis with advanced interactivity
Scientific Reports (2022)
-
Reciprocal regulation of cellular mechanics and metabolism
Nature Metabolism (2021)
-
Cell monolayers sense curvature by exploiting active mechanics and nuclear mechanoadaptation
Nature Physics (2021)